|
Note: Large images and
tables on this page may necessitate printing in landscape mode.
Copyright
©2006 The McGraw-Hill Companies. All rights reserved.
Emergency
Medicine Atlas > Part 1. Regional
Anatomy > Chapter 2. Ophthalmologic Conditions >
|
Neonatal Conjunctivitis (Ophthalmia Neonatorum)
Associated Clinical Features
Neonatal conjunctivitis is
acquired either during birth with passage through the mother's cervix and
vagina or from cross infection in the neonatal period. Microbiologic
etiologies include Chlamydia trachomatis, viruses (herpes
simplex), and bacteria (Neisseria gonorrhoeae, Staphylococcus aureus,
Streptococcus pneumoniae, groups A and B streptococci, Haemophilus
species, Pseudomonas aeruginosa, and Escherichia coli). Of
these, S. aureus is the most frequent and N. gonorrhoeae
the most important. Clinical findings in neonatal conjunctivitis include
drainage, conjunctival hyperemia, chemosis, and lid edema.
Neisseria gonorrhoeae
presents as a hyperacute bilateral conjunctivitis. Distinctive findings
include a copious purulent drainage (Fig. 2.1) and preauricular
adenopathy. The incubation period, like that for sexually transmitted N.
gonorrhoeae, is 3 to 5 days, although the onset may vary. In
chlamydial conjunctivitis, the incubation period is 5 to 12 days.
Clinical features of chlamydial conjunctivitis include unilateral
conjunctivitis and concomitant otitis media or pneumonia. Herpes simplex
conjunctivitis generally begins 2 to 14 days after birth; fluorescein
staining demonstrates epithelial dendrites.
|
|
|
|
|
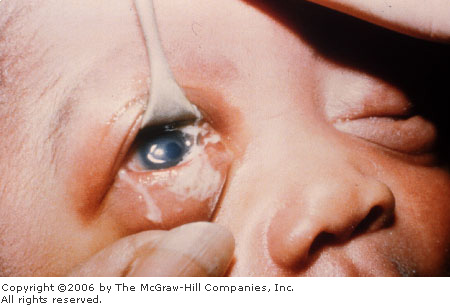
Neonatal
Conjunctivitis (Ophthalmia Neonatorum) Copious purulent drainage in a newborn with
neonatal gonococcal conjunctivitis. (Reprinted with permission of the
American Academy of Ophthalmology, Eye Trauma and Emergencies: A
Slide-Script Program. San Francisco, 1985.)
|
|
Differential Diagnosis
Dacryocystitis, corneal
abrasions, foreign body, and an obstructed nasolacrimal duct present with
redness and tearing. Neonatal glaucoma may also be mistaken for
conjunctivitis; findings include eye pain, photophobia, corneal haze,
corneal enlargement, and excessive tearing.
Emergency Department Treatment
and Disposition
The neonate with conjunctivitis
must be evaluated carefully for systemic involvement. With any form of
neonatal conjunctivitis, smears and cultures are mandatory and therapy
should begin immediately thereafter. Scrapings of the palpebral
conjunctiva for cultures and Gram stain are more revealing than
examination of the discharge itself. Topical therapy for a neonate whose
Gram stain demonstrates gram-negative diplococci includes aqueous
penicillin (10,000 to 20,000 U/mL one drop every hour for 6 to 12 h,
followed by one drop every 2 to 3 h until resolution). Saline irrigation
of the conjunctival cul-de-sac prior to antibiotic instillation may be
helpful. Systemic therapy involves intravenous penicillin G (50,000
U/kg/day in two or three doses for 7 days). Pediatric consultation is
advised.
Chlamydial conjunctivitis is
treated with oral erythromycin estolate. Neonatal bacterial conjunctivitis
that is neither gonococcal nor chlamydial may be treated with antibiotic
ointment (erythromycin, tetracycline, or gentamicin four to six times a
day for 2 weeks) and should be reevaluated in 24 h. Herpes simplex
conjunctivitis is treated with intravenous acyclovir and topical
trifluorothymidine.
Evaluation of the newborn's
parents should be undertaken in neonatal conjunctivitis due to N. gonorrhoeae,
Chlamydia, or herpes simplex virus.
Clinical Pearls
1. Neonatal conjunctivitis may
be caused by chemical, chlamydial, viral, and bacterial agents.
2. The "rule of
fives" is fairly accurate in predicting the most likely bacterial
etiology.
|
0 to 5 days:
|
N.
gonorrhoeae
|
|
5 days to
5 weeks:
|
Chlamydia
|
|
5 weeks to
5 years:
|
Streptococcus or Haemophilus
influenzae
|
|
|
|
3. The cornea should be
examined for involvement. Corneal ulcers, perforation, permanent
scarring, and blindness can quickly result from gonococcal eye infection
in the neonate. It is one of the few urgent conjunctival infections.
4. A detailed maternal history
may help with the diagnosis of neonatal conjunctivitis secondary to N.
gonorrhoeae, Chlamydia, or herpes.
|
|
Bacterial Conjunctivitis
Associated Clinical Features
Bacterial conjunctivitis is
characterized by the acute onset of conjunctival injection and
mucopurulent drainage. Staphylococcus aureus is the most common
causative bacterium. Streptococcus pneumoniae and Haemophilus
influenzae occur more frequently in children. The purulent drainage
(Fig. 2.2) commonly leads the eyelids to be stuck together on awakening.
Lid edema and erythema, chemosis, and superficial punctate keratitis may
also be present.
|
|
|
|
|
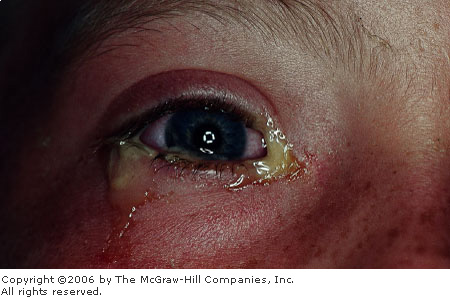
Bacterial
Conjunctivitis Mucopurulent
discharge, conjunctival injection, and lid swelling in a 10-year-old
with Haemophilus influenzae conjunctivitis. (Courtesy of Frank
Birinyi, MD.)
|
|
The most severe form of acute
purulent conjunctivitis is associated with Neisseria gonorrhoeae.
Infection can be seen in children of any age, including the newborn.
Clinical symptoms are hyperacute in onset and include a discharge that is
prominent, thick, copious, and purulent. Other findings include marked
eyelid swelling, along with tenderness, marked conjunctival hyperemia,
pain, chemosis, and preauricular adenopathy. The condition may progress
to involve the cornea, because Neisseria species are capable of
invading an intact corneal epithelium. Corneal findings include a diffuse
epithelial haze, epithelial defects, marginal infiltrates, and peripheral
ulcerative keratitis, which can rapidly progress to perforation.
Neonatal conjunctivitis is
discussed separately.
Differential Diagnosis
Other etiologies of
conjunctivitis (viral, allergic) as well as iritis, glaucoma, scleritis,
and foreign body also present as a red eye.
Emergency Department Treatment
and Disposition
Treatment involves local hygiene
with warm, moist compresses and frequent hand washing, along with broad-spectrum
antibiotic drops (10% sulfacetamide, gentamicin, ofloxacin,
ciprofloxacin, or trimethoprim/polymyxin B). One drop every 3 h for 7 to
10 days usually results in rapid resolution. If there is no response,
cultures and ophthalmologic consultation should be sought.
The treatment of gonococcal
conjunctivitis is both systemic (ceftriaxone 25 to 50 mg/kg/day, not to
exceed 4 g/day, given in one dose IM for 7 days) and topical (penicillin
G 100,000 U/mL one drop every 2 h or bacitracin ophthalmic ointment 500
U/g every 2 h, tapering over 48 h to five times a day). Patients with
corneal involvement should receive additional intravenous ceftriaxone (1
g every 12 h). Sexual partners should be advised and evaluated.
Clinical Pearls
1. Worsening symptoms during topical
treatment with any antibiotic, particularly Neosporin or a sulfonamide
(Sodium Sulamyd), may represent a contact allergic reaction.
2. Conjunctivitis due to N.
gonorrhoeae must be considered in the sexually active adult with a
prominent, thick, copious, and purulent eye discharge.
3. Neisseria species are
capable of invading an intact corneal epithelium.
4. In early or mild cases of
bacterial conjunctivitis, symptoms may be limited to mild conjunctival
injection without frankly purulent drainage that is evident to the
physician. Thus, empiric antibiotic therapy is warranted in most cases of
conjunctivitis.
|
|
Viral Conjunctivitis
Associated Clinical Features
Viral conjunctivitis is an
infection caused most commonly by adenoviruses. Clinical features range
in severity but usually are mild and typically include burning or
irritation, conjunctival injection, lid edema, chemosis, and a thin,
watery discharge. The infection usually begins in one eye, but both eyes
generally become involved because of autoinoculation (Fig. 2.3). The
palpebral conjunctiva may demonstrate hyperemia and follicles, which are
hyperplastic lymphoid tissue appearing as gray or white lobular
elevations. The palpebral conjunctiva also may demonstrate papillae.
Papillae are seen in many acute inflammatory diseases, secondary to a
hyperplastic conunctival epithelium being thrown into numerous folds and
projections. Clinically, papillae give the palpebral conjunctiva a
velvety appearance. Preauricular adenopathy may be present. Severe cases
may demonstrate focal or diffuse subconjunctival hemorrhages as well as
pseudomembranes. A punctate keratitis may appear several days after the
onset of symptoms, followed several weeks later by subepithelial
infiltrates. The visual acuity and pupillary reactivity are normal.
|
|
|
|
|
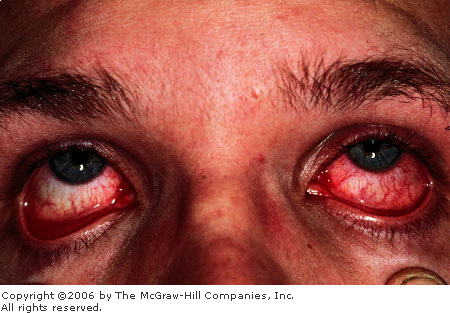
Viral
Conjunctivitis Note the
classic asymmetric conjunctival injection. Symptoms first developed
in the left eye, with symptoms spreading to the other eye a few days
later. A thin watery discharge is also seen. (Courtesy of Kevin J.
Knoop, MD, MS.)
|
|
Pharyngoconjunctival fever,
usually caused by adenovirus type 3, is highly infectious and should be
considered if there is fever, upper respiratory tract infection (cold,
flu, or sore throat), and preauricular adenopathy. It is seen
predominantly in the young and institutionalized, with epidemics
occurring in families, schools, and military camps.
Epidemic keratoconjunctivitis is
discussed separately.
Differential Diagnosis
Similar symptoms are seen in
allergic and bacterial conjunctivitis and other causes of a red eye, such
as scleritis, glaucoma, and iritis. Less common diagnoses include preseptal
cellulitis and dacryoadenitis.
Emergency Department Treatment
and Disposition
Many cases are self-limited and
mild. Cool compresses are helpful. Meticulous hygiene (hand washing by
the family and instrument cleaning by medical personnel) is necessary to
prevent spread. Because the signs and symptoms of viral conjunctivitis do
not always suffice to distinguish it from bacterial conjunctivitis,
antibacterial eye drops are usually prescribed. Antivirals are
ineffective against adenovirus. Topical steroids should be avoided.
Symptoms may persist for several weeks; follow-up is indicated if
symptoms have not begun to resolve within 4 to 7 days.
Clinical Pearls
1. Adenoviruses are the most
common cause of acute conjunctivitis. However, a complete eye examination
is necessary to rule out other more serious causes of a red eye before
the diagnosis of viral conjunctivitis is made.
2. Adenovirus most commonly
demonstrates a follicular conjunctivitis. Papillae may also be seen on
the palpebral conjunctiva.
3. Pharyngoconjunctival fever
should be considered in the setting of fever, upper respiratory tract
infection, and conjunctivitis.
|
|
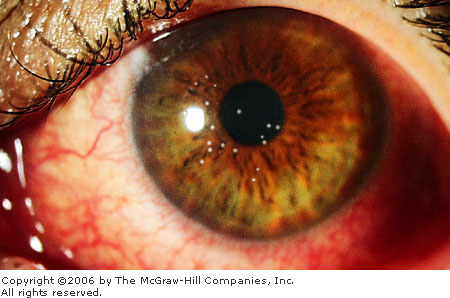
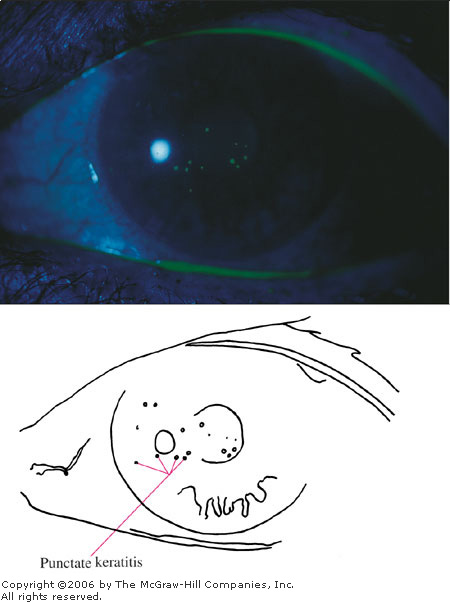
Epidemic Keratoconjunctivitis
Associated Clinical Features
Epidemic keratoconjunctivitis
(EKC) is a severe and highly contagious adenovirus infection involving
the conjunctiva and cornea. Viral transmission usually occurs through
direct or indirect contact with the ocular secretions of infected
individuals. The incubation period after exposure is about 8 days.
Initial symptoms include watery or mucopurulent discharge, foreign-body
sensation, and mild photophobia. Clinical findings include edema of the
eyelids, chemosis, marked diffuse conjunctival hyperemia, subconjunctival
hemorrhage, and a follicular and papillary conjunctival reaction (Fig.
2.4). Pseudomembranes overlying the palpebral conjunctiva and tender
preauricular nodes may be present. A painful keratitis, typically
involving the central cornea, develops in about 80% of patients, usually
around the eighth day. The keratitis initially appears as fine punctate
epithelial lesions that stain with fluorescein (Figs. 2.5, 2.6). These
lesions coalesce and continue to stain with fluorescein. By the end of
the second week, the keratitis is replaced by white macular,
subepithelial infiltrates located in the central cornea. These lesions no
longer stain with fluorescein. They may cause a significant decrease in
vision and photophobia for months and even years, but they eventually
resolve spontaneously. These subepithelial infiltrates are believed to
result from a host immune response rather than from active viral
replication.
|
|
|
|
|
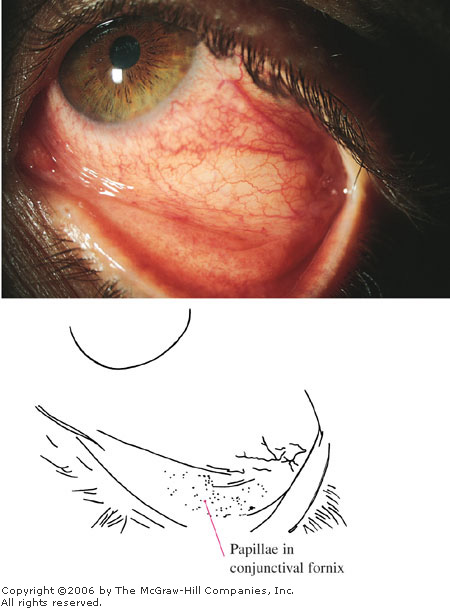
Epidemic
Keratoconjunctivitis (EKC)
Diffuse injection of the bulbar conjunctiva is seen in addition to a
papillary reaction of the palpebral conjunctiva—a classic
finding in EKC. (Courtesy of Katrina C. Santos.)
|
|
|
|
|
|
|
Epithelial
Keratitis This usually
develops after 5 to 7 days and is seen as a fine punctate abrasion
pattern over the cornea. (Courtesy of Katrina C. Santos.)
|
|
|
|
|
|
|
Fluorescein
Stain This demonstrates
epithelial keratitis from the prior photo. (Courtesy of Katrina C.
Santos.)
|
|
Symptoms usually begin
unilaterally in young adults during the fall and winter months. Bilateral
involvement may develop 4 to 5 days later, with less severe symptoms in
the second eye, probably due to partial immune protection of the host.
There are few to no systemic complaints. Thus, in patients with systemic
complaints and associated fever, upper respiratory tract infection,
pharyngitis, otitis media, and diarrhea, the diagnosis is more likely to
be pharyngoconjunctival fever, which is also caused by an adenovirus.
Pharyngoconjunctival fever is seen predominately in the young, with
epidemics occurring in families and schools.
Differential Diagnosis
Other viruses and causes of red
eye (bacterial conjunctivitis, iritis, scleritis, glaucoma, herpetic
infection) need be considered.
Emergency Department Treatment
and Disposition
Although EKC may be severe, the
condition is self-limited and therapy is mainly palliative. Cool
compresses, topical vasoconstrictors (Vasocon), and dark sunglasses
provide symptomatic relief. Antibiotics and antivirals are ineffective.
However, topical broad-spectrum antibiotic drops are usually prescribed,
since it is difficult to distinguish viral from bacterial conjunctivitis.
In the presence of pseudomembrane formation, topical broad-spectrum
antibiotic ointments may be prescribed to lubricate and protect the
cornea. The use of topical corticosteroids is controversial. They may
have a role in patients with marked symptoms such as severe conjunctival
pseudomembrane formation, severe foreign-body sensation, or reduced
visual acuity secondary to epithelial or subepithelial keratitis.
Although topical steroids do provide effective symptomatic relief, they
have no beneficial therapeutic effect on the ultimate clinical outcome.
Patients with EKC should limit their exposure to others for 2 weeks after
the onset of the disease and use separate linens.
Clinical Pearls
1. A nonspecific adenoviral
conjunctivitis will resolve in 10 to 14 days; a virulent adenovirus
causing EKC will peak in 5 to 7 days and may last 3 to 4 weeks.
2. Frequent hand washing and
the use of separate linens is advised for patients and family members to
reduce exposure.
3. Pharyngoconjunctival fever
should be considered if there is an associated fever and upper
respiratory tract infection.
4. EKC may be nosocomially
transmitted by tonometry (the footplate of the Schiøtz tonometer, the
prism of the applanation tonometer), contaminated solutions (topical
anesthetics), and the physician's fingers. Regular hand washing by the physician
and patient and careful cleaning (using alcohol or Dakin's solution
followed by rinsing) and sterilization of instruments are therefore
important. Adenovirus can be recovered for extended periods of time from
these surfaces.
|
|
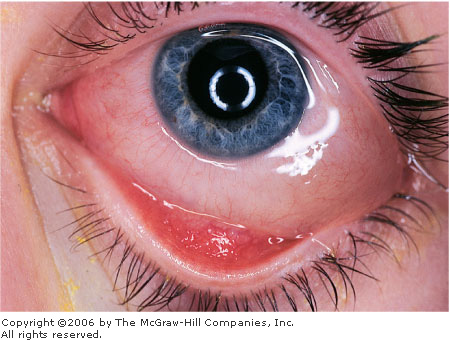
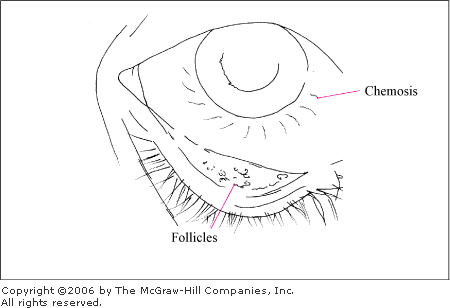
Allergic Conjunctivitis
Associated Clinical Features
Allergic conjunctivitis is a
recurrent condition whereby airborne allergens precipitate
hypersensitivity reactions in the conjunctiva. Potential allergens
include pollens (ragweed, grasses, trees, weeds), animal dander, mold,
and dust. Itching is the hallmark symptom. Allergic conjunctivitis is
usually transient and self-limited and is seasonal if it is due to
pollens. However, it can present as a single acute episode if the
allergen is animal dander, mold, or dust. There may be a personal or
family history of atopy, eczema, asthma, and allergic rhinitis (hay
fever). Ocular allergic conditions are more common in males and tend to
commence in the first or second decade of life.
In addition to the hallmark
symptom of itching, associated clinical features include conjunctival
injection and edema, burning, discharge (clear, white, or mucopurulent),
and chemosis (swelling of the bulbar conjunctiva). Chemosis may be marked
and may actually balloon beyond the lids. Small to medium-sized papillae
(hyperplastic conjunctival epithelium thrown into numerous folds and
projections) appear as small elevations on the palpebral conjunctiva
(Fig. 2.7) and give the tissue a velvety appearance. Pallor of the
palpebral conjunctiva may be present due to edema. The eyelids may be red
and swollen.
|
|
|
|
|
|
|
Allergic
Conjunctivitis Conjunctival
injection, chemosis, and a follicular response in the inferior
palpebral conjunctiva in this patient with allergic conjunctivitis
secondary to cat fur. (Courtesy of Timothy D. McGuirk, DO.)
|
|
Vernal conjunctivitis is an infrequent but serious
form of allergic conjunctivitis that mainly affects young males (4 to 16
years of age) during the warm months or in tropical climates. Again, a
family or personal history of atopy is common. In addition to a
hereditary predisposition, exogenous factors play a role in the severity
and likelihood of this disease. The Middle East and North Africa, with
arid areas and wind and dust storms, have the highest incidence of vernal
conjunctivitis. Symptoms of vernal conjunctivitis are similar to those of
allergic conjunctivitis but greater in degree. Itching is intense, and a
vigorous knuckle rubbing is a typical observation. Giant, raised,
pleomorphic papillae ("cobblestones") are seen over the upper
tarsal plate (but rarely over the lower tarsal plate) and are
pathognomonic for the disease (Fig. 2.8). The drainage of vernal
conjunctivitis is also pathognomonic and is characteristically tenacious,
copious, thick, and ropy. Corneal findings include a sterile ulcer (well
delineated with an oval or shield shape and no surrounding haze or
iritis), Horner-Trantas dots (raised white limbal infiltrates), and
superficial punctate keratopathy.
|
|
|

|
|
Vernal
Conjunctivitis The tarsal
conjunctiva demonstrates giant papillae and a cobblestone appearance
pathognomonic for vernal conjunctivitis. (Courtesy of William Beck.)
|
|
Differential Diagnosis
Other etiologies of a red eye
(scleritis, iritis, glaucoma, bacterial conjunctivitis) should be
considered.
Emergency Department Treatment
and Disposition
The severity of the allergic
condition is directly proportional to the level and duration of the
allergen exposure. Therefore, initial therapy is primarily aimed at
identification and elimination of the allergen. Avoiding animal dander,
using air conditioners with appropriate filters, limiting time outdoors (to
avoid pollen-bearing wind), or the use of goggles or glasses outdoors
will improve the condition.
Topical tear substitutes (four to
eight times a day) are effective in diluting or washing away the
allergen. For mild allergic conjunctivitis, H1 antihistamine-vasoconstrictor
combinations successfully alleviate itching, redness, and swelling.
Vasocon-A (one to two drops every 3 to 4 h) is effective and contains an
antihistamine (0.5% antazoline) and a vasoconstrictor
(0.05% naphazoline). Olopatadine (0.1%, one to two drops bid) is an
antihistamine with mast cell–stabilizing properties that also
relieves the itching and redness effectively. Mild topical steroids are
an option after consultation with an ophthalmologist for those cases
where all other modalities have been explored. A short course of
prednisolone (0.12%) two to three times a day is unlikely to result in
any ocular complications.
Additional therapeutic agents for
vernal conjunctivitis include cromolyn sodium solution 4% (one to two
drops every 4 to 6 h), aspirin (650 mg four times a day orally), and cold
compresses. Topical cyclosporine (0.5%) may be useful in resistant cases
of vernal conjunctivitis.
Clinical Pearls
1. Itching is the hallmark
symptom of ocular allergy.
2. Removal of the offending allergen,
if possible, is the first step in the treatment of allergic
conjunctivitis. The patient is usually the best source for identifying
the allergen to which he or she is sensitive.
3. Vasocon-A and olopatadine
are effective in treating allergic conjunctivitis.
4. Topical corticosteroids may
be used in severe cases but should be prescribed only with ED
ophthalmology consultation. Complications include glaucoma, cataract
formation, secondary infection, and corneal perforation.
5. Vernal conjunctivitis
affects mainly children and adolescents; it peaks in the warmer months.
6. Evert the upper lid to
appreciate the giant conjunctival papillae in vernal conjunctivitis.
Their cobblestone appearance is pathognomonic.
|
|
Hordeolum/Chalazion
Associated Clinical Features
A hordeolum is an acute purulent
infection and a localized abscess involving the meibomian glands, the
glands of Zeis, or the glands of Moll (Fig. 2.9). Staphylococcus
aureus is the most frequent isolate. An external hordeolum (stye)
involves an eyelash follicle and the adjacent glands of Zeis or Moll
(Fig. 2.10). An internal hordeolum involves the meibomian glands within
the tarsal plate. A chalazion is a chronic localized lipogranulomatous
inflammation that results from the obstruction of the meibomian glands. A
chalazion may evolve from a hordeolum but usually arises spontaneously
secondary to blockage or obstruction of the gland. The impacted
secretions of the gland are then extruded into the surrounding tissues,
producing a foreign-body reaction to sebum and a lipogranulomatous
inflammation. Chalazia are commonly seen in the ED (Fig. 2.11).
|
|
|
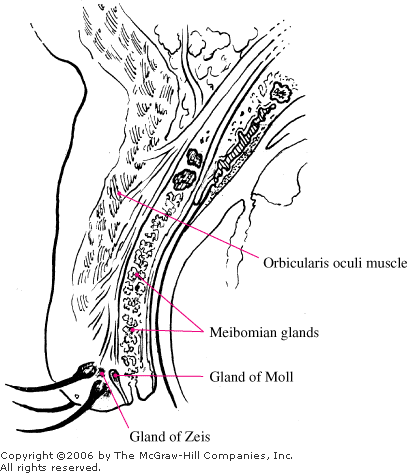
|
|
Eyelid
Anatomy Anatomic structures
related to eyelid pathology.
|
|
|
|
|
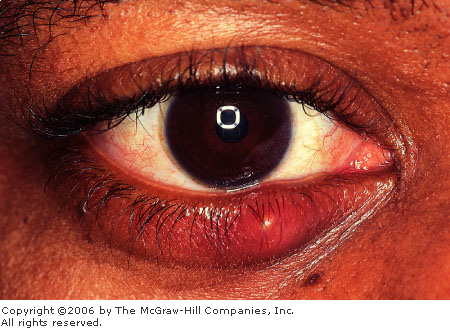
|
|
Hordeolum Focal swelling and erythema at the lid margin
are seen in this hordeolum. (Courtesy of Frank Birinyi, MD.)
|
|
|
|
|
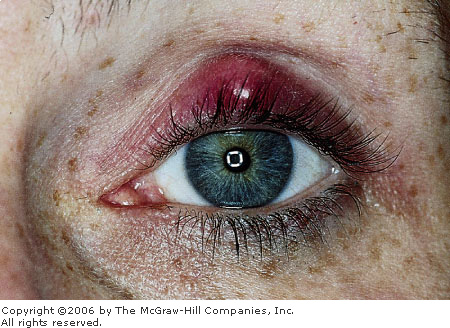
|
|
Chalazion This chalazion shows nodular focal swelling
and erythema. (Courtesy of Frank Birinyi, MD.)
|
|
Common signs and symptoms include pain, focal
swelling, edema, erythema, and tenderness. In the case of an external
hordeolum (stye), an abscess localizes around the root of an eyelash,
followed by necrosis of the skin and spontaneous evacuation of the
abscess at the lid margin. An internal hordeolum, produced by obstruction
of the meibomian duct with associated bacterial infection, demonstrates a
localized inflammation of the tarsal plate. In the case of a chalazion,
focal inflammation may cause pointing of the lesion either anteriorly
(toward the skin of the eyelid) or posteriorly (toward the tarsal
conjunctiva) (Figs. 2.12, 2.13). A chalazion may become sufficiently
large as to press on the globe and cause astigmatism (corneal distortion
that prevents focus). A chronic chalazion may appear as focal lid
swelling without associated signs of inflammation.
|
|
|
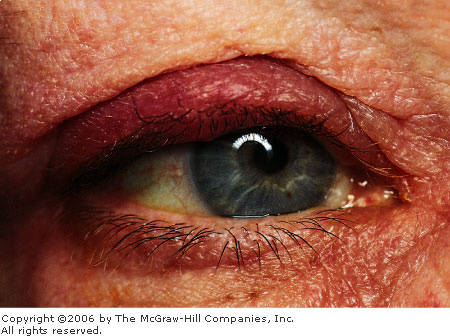
|
|
Chalazion This chalazion is in an early stage. Lid
swelling is evident, with pointing of the chalazion to the inner
tarsal conjunctiva. (Courtesy of Kevin J. Knoop, MD, MS.)
|
|
|
|
|
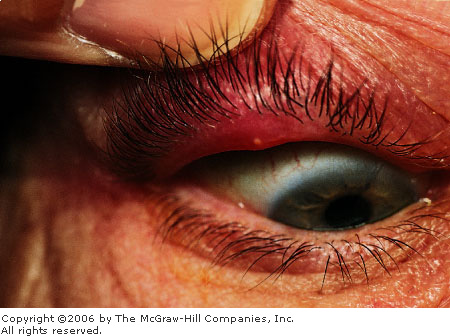
|
|
Chalazion Pointing of the chalazion to the tarsal
conjunctiva is more evident with slight lid eversion. (Courtesy of
Kevin J. Knoop, MD, MS.)
|
|
There may be associated marginal blepharitis (Fig.
2.14) (a chronic low-grade inflammation of the lid margins with crusts
around the lashes) or acne rosacea (a dermatologic condition with facial
hyperemia, acneiform lesions, hypertrophy of sebaceous glands, and
rhinophyma).
|
|
|
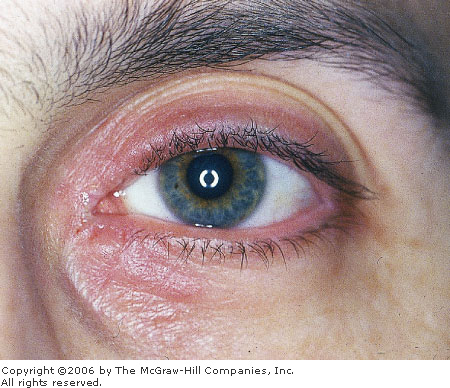
|
|
Blepharitis Inflamed, erythematous eyelid margins
consistent with blepharitis. (Courtesy of Kevin J. Knoop, MD, MS.)
|
|
Differential Diagnosis
Sebaceous cell or squamous cell
carcinoma should be suspected in older patients with recurrent or
persistent lesions. Preseptal cellulitis should be considered if the
entire lid is erythematous and edematous. Pyogenic granuloma has a
similar appearance but includes hypertrophic tissue as well as a vascular
core, and it bleeds easily. Dacryocystitis should be considered if the
lesion involves the medial aspect of the lower lid.
Emergency Department Treatment
and Disposition
The clinical course of a
hordeolum may be self-limited, with spontaneous drainage of the abscess
and resolution within 5 to 7 days. Treatment for both hordeola and
chalazia includes warm compresses 2 to 4 times a day for 15 min. Warm
compresses help to localize the infection and inflammation and may
expedite spontaneous drainage. Systemic antibiotics are unnecessary
unless there is a significant cellulitis. Topical antibiotic ointment
cannot directly affect the inflammation inside the gland but is an
adjunctive therapy to decrease the local bacterial flora. The application
of a broad-spectrum antibiotic ointment, such as bacitracin or
erythromycin, every 3 h to the conjunctival sac is effective. If the mass
persists beyond 3 to 4 weeks or if the lesion is sufficiently large to
distort vision, referral to the ophthalmologist should be made for
incision and curettage or intralesional corticosteroid injection. Gentle
scrubbing of the eyelids and lashes may be indicated if marginal blepharitis
is noted.
Clinical Pearls
1. Chalazia are often found in
patients with marginal blepharitis, probably because the orifices of the
meibomian gland are blocked by the blepharitis infection.
2. Excisional biopsy is
indicated for recurrent chalazia to exclude malignancy.
|
|
Dacryocystitis
Associated Clinical Features
Dacryocystitis, an inflammation
of the lacrimal sac, is usually secondary to obstruction of the
nasolacrimal duct. Hallmark findings are tearing (epiphora) and
discharge. Acute dacryocystitis is associated with pain, swelling over
the lacrimal sac (Fig. 2.15), erythema, and tenderness. Mucopurulent
discharge may be expressed from the punctum when pressure is applied over
the lacrimal sac. In adults, acute infection is due to Staphylococcus
aureus or occasionally beta-hemolytic streptococci.
|
|
|
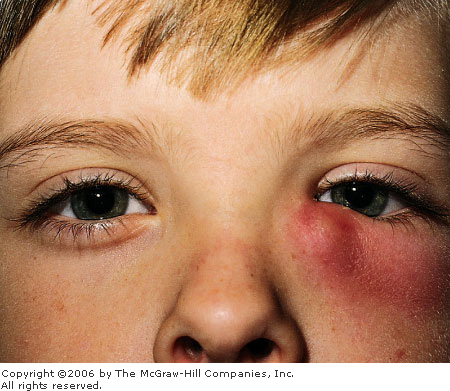
|
|
Dacryocystitis Swelling and erythema over the medial lid and
lacrimal sac developed in this 10-year-old patient with streptococcal
pharyngitis. (Courtesy of Kevin J. Knoop, MD, MS.)
|
|
In the newborn, 4 to 7% have a
closed nasolacrimal passage. In these cases the duct usually opens
spontaneously within the first month. Dacryocystitis is uncommon but may
nevertheless develop, and aggressive treatment is required to avoid
orbital cellulitis. Haemophilus influenzae is the most common
organism isolated. Organisms usually seen in chronic dacryocystitis
include Streptococcus pneumoniae or, rarely, Candida albicans.
Dacryocystitis is uncommon in the
intermediate age groups unless it follows chronic sinusitis, facial
trauma, or (rarely) neoplasm.
Differential Diagnosis
Chalazion, facial and orbital
cellulitis, canaliculitis, canalicular stenosis, sinus tumors, ethmoid
sinusitis, and mucoceles have similar features.
Emergency Department Treatment
and Disposition
Acute dacryocystitis usually
responds to oral antibiotics (amoxicillin-clavulanate), warm compresses,
and gentle massage. Topical antibiotics may be used in chronic cases.
Nonurgent ophthalmology or otolaryngology referral is required for
definitive treatment—relief of the obstruction by
dacryocystorhinostomy. In febrile and ill-appearing patients with acute
dacryocystitis, hospitalization for intravenous antibiotics (cefuroxime)
is indicated.
In infants with chronic
dacryocystitis, both topical and oral antibiotics may be used. Referral
is indicated if signs do not regress by 6 to 9 months of age or if acute
dacryocystitis develops.
Clinical Pearls
1. The swelling is localized to
the extreme nasal aspect of the lower lid and is usually unilateral.
2. The diagnosis of acute
dacryocystitis may be confirmed by pressure on the lacrimal sac and the
expression of purulent material from the punctum. The lacrimal sac and
lacrimal fossa are situated in the inferior medial aspect of the orbit,
not on the side of the nose.
3. The incidence follows a
bimodal distribution. Dacryocystitis usually occurs in infants or persons
over 40 years of age.
|
|
Dacryoadenitis
Associated Clinical Features
Dacryoadenitis is an uncommon
condition involving inflammation of the lacrimal gland. Most acute cases
are associated with systemic infection, although this may not be readily
apparent. Findings are localized to the outer one-third of the upper
eyelid and include fullness or swelling, conjunctival chemosis, and
injection laterally (Fig. 2.16), erythema, and tenderness. A
characteristic "S"-shaped deformity with mechanical ptosis of the
upper lid is seen. The lacrimal gland may be palpable. Painful
ophthalmoparesis and diplopia may be present secondary to involvement of
the adjacent lateral rectus muscle.
|
|
|
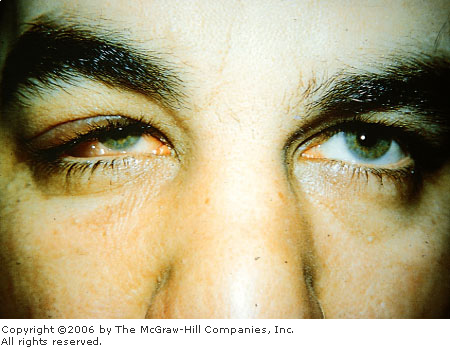
|
|
Dacryoadenitis Unilateral localized swelling and chemosis are
present laterally secondary to inflammation of the lacrimal gland.
(Used with permission from the American Academy of Ophthalmology: External
Disease and Cornea: A Multimedia Collection. San Francisco,
1994.)
|
|
Conditions associated with
dacryoadenitis include sarcoidosis and Sjögren's syndrome. Infectious
causes are more frequent; they include gonorrhea and mumps. Lacrimal
gland masses, including lymphoma and epithelial tumors, may be
neoplastic.
Differential Diagnosis
Chalazion, conjunctivitis,
preseptal cellulitis, orbital cellulitis, and lacrimal gland tumor are
other conditions to consider.
Emergency Department Treatment
and Disposition
In the setting of acute bacterial
infection, oral antibiotics (amoxicillin-clavulanate) are given for mild
to moderate cases. In moderate to severe infections, intravenous
antibiotics (ticarcillin-clavulanate) may be necessary. Viral
dacryoadenitis (mumps) is treated with cool compresses and analgesics
(acetaminophen); it resolves spontaneously without sequelae. Nonemergent
ophthalmology follow-up is appropriate. Patients should be instructed to
return to the ED urgently for symptoms suggestive of orbital cellulitis
(decreased ocular motility or proptosis).
Clinical Pearls
1. The swelling is usually
unilateral and localized over the lateral one-third of the upper lid. It
imparts an "S"-shaped curve to the lid margin.
2. In children, acute
dacryoadenitis is most often seen as a complication of mumps with
accompanying bilateral parotid swelling.
3. Approximately half of
lacrimal gland masses are inflammatory; the other half are neoplastic.
|
|
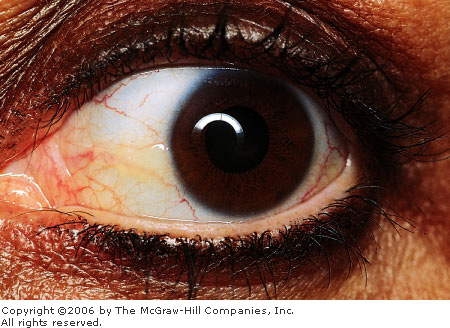
Pinguecula/Pterygium
Associated Clinical Features
A pinguecula (Latin: pinguecula
meaning fatty) is a common degenerative lesion of the bulbar conjunctiva.
It appears as a light brown or yellow-white amorphous conjunctival tissue
adjacent to the limbus, usually nasally (Fig. 2.17). A pinguecula is
usually asymptomatic and generally does not require treatment. It may
become episodically inflamed, gradually enlarge over time, or become a
pterygium.
|
|
|
|
|
Pinguecula A small area of yellowish "heaped
up" conjunctival tissue is seen adjacent to the limbus on the
nasal aspect. (Courtesy of Kevin J. Knoop, MD, MS.)
|
|
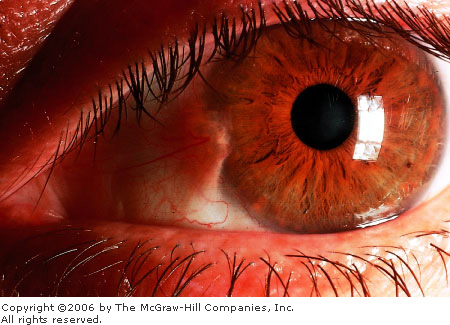
A pterygium (Greek: pterygion meaning a
wing-like thing) is a benign proliferation of fibrovascular tissue. It
originates within the bulbar conjunctiva and extends onto the peripheral
cornea. Early in its course a pterygium may be indistinguishable from a
pinguecula. It may then enlarge to progressively encroach onto the cornea
and visual axis. Typically a pterygium assumes a triangular
configuration, with the apex of the lesion directed toward the pupil
(Fig. 2.18). Growth occurs from this apex. Risk factors include exposure
to ultraviolet light (sunlight), wind, and dust. Pterygia and pingueculae
are generally seen in older individuals living in warmer areas with high
levels of sunlight. Like pingueculae, pterygia are more likely to involve
the nasal portion of the bulbar conjunctiva. Pterygia may be asymptomatic
or become inflamed, giving rise to mild symptoms of irritation and
foreign-body sensation. Decreased visual acuity may develop if the visual
axis is involved or if the lesion induces astigmatism (irregular corneal
curvature resulting in refractive error).
|
|
|
|
|
Pterygium This pterygium appears as a raised vascular
triangular area of bulbar conjunctiva that encroaches on the cornea.
(Courtesy of the Department of Ophthalmology, Naval Medical Center,
Portsmouth, VA.)
|
|
Differential Diagnosis
A pseudopterygium may have a
similar appearance. A pseudopterygium is a fibrovascular scar arising in
the bulbar conjunctiva and extending onto the cornea. It is the result of
previous ocular inflammation (e.g., chemical burns, trauma, and
infection). Episcleritis, corneal ulcer, and conjunctival neoplasm should
be considered in the differential diagnosis.
Emergency Department Treatment
and Disposition
A patient with mild disease can
be treated with artifical tears or a topical vasoconstrictor (Vasocon).
In more severe cases, topical steroids may be prescribed after
consultation. Nonemergent referral to an ophthalmologist is appropriate.
Excision of a pinguecula (or pterygium) is indicated if the lesion
interferes with contact lens wear, becomes chronically inflamed, or
constitutes a cosmetic problem. Pterygia are excised if they cause
persistent discomfort, encroach significantly on the cornea to involve
the visual axis, or restrict the movement of extraocular muscles.
Clinical Pearls
1. Pterygia and pingueculae are
usually found on the nasal conjunctiva, adjacent to the limbus, in the
horizontal meridian.
2. Pterygia are a particular
problem in sunny, hot, dusty regions. Eye protection (goggles,
sunglasses) helps to reduce the irritation.
|
|
Scleritis
Associated Clinical Features
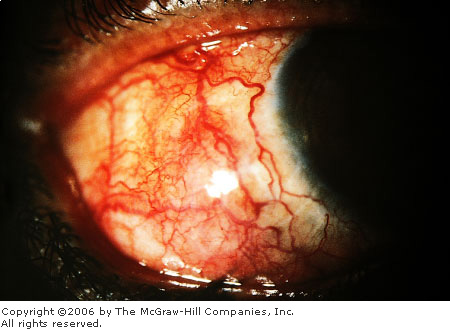
Scleritis is a destructive and
serious inflammation involving the sclera. The sclera is the tough,
flexible white outer covering of the eye, composed of collagen and
elastic fibers. The onset of scleritis is gradual. Pain, tearing, and
photophobia are prominent features. The pain, frequently severe, may
awaken the patient at night. It may radiate to the forehead, temple,
brow, or jaw. Ocular movement is usually painful.
Patients with scleritis have an
intensely red eye with a violaceous or purple hue, secondary to
engorgement of the deep vessels of the episclera (Fig. 2.19). The blood
vessels of the conjunctiva and superficial episclera are also commonly
involved. Sectorial (versus diffuse) scleritis may mimic episcleritis.
However, in scleritis, the globe is tender to palpation and the deep
episcleral vessels do not move when the overlying tissues are moved with
a cotton-tipped applicator, nor do they blanch with topical phenylephrine
(2.5%). The normal radial vascular pattern of the episcleral vessels is
lost. Scleral edema may be seen with slit-lamp biomicroscopy. (After the
topical application of phenylephrine, the slit-lamp light beam appears to
bow forward as the beam makes its excursion across the scleral surface.)
Corneal involvement, iritis (with cells and flare in the anterior
chamber) and decreased visual acuity frequently accompany scleritis. In
patients with a previous history of scleritis, the uveal layer may be
visible ("uveal show") in areas where scleral tissue has been
lost.
|
|
|
|
|
Scleritis A prominent generalized vascular injection is
present. These vessels do not move when the overlying conjunctiva is
moved with a cotton-tipped applicator. (Courtesy of Thomas F. Mauger,
MD.)
|
|
Scleritis is associated with a
number of autoimmune and infectious conditions; rheumatoid arthritis is
the most common. Scleritis occurs more frequently in women and in the
fourth to sixth decades of life.
Differential Diagnosis
Other causes of a red eye
(conjunctivitis, iritis, episcleritis, trauma, glaucoma) should be
considered. Scleritis may be confused with an inflamed pinguecula,
pterygium, foreign body, or tumor.
Emergency Department Treatment
and Disposition
Ophthalmology consultation and
systemic therapy are required. Oral nonsteroidal anti-inflammatory drugs
(NSAIDs) are recommended. Systemic steroids are added if oral NSAIDs are
ineffective or for severe scleritis. Topical steroids and NSAIDs are
occasionally effective. Immunosuppressive therapy (cyclophosphamide,
methotrexate, azathioprine) is sometimes required, especially for
progressive cases. Appropriate treatment of associated systemic
autoimmune disease is also important. The intraocular pressure should be
measured, since secondary glaucoma is associated with scleritis.
Clinical Pearls
1. Scleritis—unlike
episcleritis, which may be self-limited—is destructive and
threatens the patient's vision.
2. The eye is usually
exquisitely tender, and patients frequently complain of severe pain.
Episcleritis, on the other hand, is rarely associated with significant
pain or tenderness.
3. Scleritis may be the
presenting sign of a systemic disease. Rheumatoid arthritis is the most
common.
|
|
Episcleritis
Associated Clinical Features
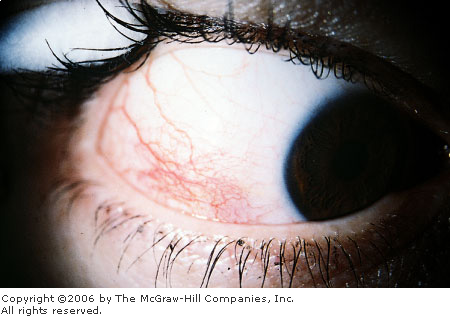
Episcleritis is a common, benign,
and frequently recurring inflammatory disease of the episclera; it
typically affects young adults. The episclera is a thin layer of vascular
elastic tissue overlying the primarily avascular sclera and is partly
responsible for scleral nutrition. Tenon's capsule, a superficial layer
within the episclera, acts as a synovial membrane for smooth movement of
the eye. Episcleral vessels are large, run in a radial direction, and can
be seen beneath the conjunctiva. Two separate vascular plexuses are found
within the episclera. The superficial episcleral plexus is inflamed in
episcleritis. These vessels blanch with the use of topical
2.5%phenylephrine drops. Vessels in the deep episcleral plexus, on the
other hand, are associated with scleritis and remain dilated after the
use of such drops.
Patients may complain of mild
pain, foreign-body sensation, mild tenderness, irritation, photophobia,
and excessive lacrimation. The affected eye appears normal except for
nodular (or, rarely, diffuse) pink or bright red conjunctival and
episcleral inflammation from dilation of the vessels in the superficial
episcleral vascular plexus (Fig. 2.20). Visual acuity is normal. There is
no history of trauma or purulent discharge.
|
|
|
|
|
Episcleritis A localized area of hyperemia consistent with
episcleritis is seen in the lower lateral quadrant of the eye.
(Courtesy of Thomas F. Mauger, MD.)
|
|
Episcleritis is usually an
isolated condition and the etiology is unknown. It has been associated
with gout, systemic lupus erythematosus, rheumatoid arthritis,
inflammatory bowel disease, and herpes zoster.
Differential Diagnosis
Other considerations include
conjunctivitis and other causes of a red eye, such as iritis, acute
glaucoma, trauma, corneal ulcer, scleritis, pinguecula, and phlyctenule
(similar to episcleritis except that a nodule is present in the center of
the lesion).
Emergency Department Treatment
and Disposition
In mild cases, the condition is
self-limited and may resolve spontaneously after 1 to 2 weeks. Artificial
tears and topical vasoconstrictors (Naphcon) may be used. In those cases
associated with rheumatoid arthritis or systemic lupus erythematosus,
oral NSAIDs are recommended. Topical NSAIDS have not been shown to be
effective. Ophthalmology referral is appropriate for confirmation and
treatment.
Clinical Pearls
1. It is important to
distinguish between episcleritis and scleritis, because the latter is an
extremely serious problem that threatens the patient's vision.
2. While other causes of a red
eye demonstrate a generalized redness, episcleritis is usually localized.
3. Dilated vessels in the
superficial episcleral plexus will blanch with topical 2.5%
phenylephrine. This test helps to distinguish between episcleritis and
scleritis.
4. Episcleritis is unilateral
two-thirds of the time and is seen in young and middle-aged adults. The
gender incidence is equal.
5. Topical corticosteroids are
not indicated in episcleritis. Although they may be effective
temporarily, their use prolongs the natural history of resolution.
|
|
Acute Angle Closure Glaucoma
Associated Clinical Features
Glaucoma comprises a
heterogeneous group of disorders causing optic nerve damage, usually in
association with elevated intraocular pressure (IOP). It is a major and
preventable cause of blindness. In angle closure glaucoma (ACG), the
elevated IOP is due to an outflow obstruction of aqueous humor from the
anterior chamber. Aqueous humor is initially produced by the ciliary body
and first enters the posterior chamber. It then passes between the
posterior surface of the iris and the anterior surface of the lens to
enter the anterior chamber. After circulating within the anterior
chamber, the aqueous humor leaves through the trabecular meshwork of the
anterior chamber angle to enter Schlemm's canal. In ACG, the peripheral
iris covers this trabecular meshwork, the angle is "closed,"
and aqueous outflow is blocked. Normal IOP is 10 to 21 mmHg; it rises to
50 to 100 mmHg in ACG.
There are several anatomic and
physiologic factors that play a role in ACG. In the most common type of
ACG, a major role is played by relative pupillary block. As the lens
slowly enlarges due to normal development and aging, more of the lens's anterior
surface makes contact with the iris's posterior surface. This increases
the relative block of aqueous humor flow from the posterior chamber
through the pupil to the anterior chamber. As a result, an increased
pressure differential develops. The increased pressure in the posterior
chamber causes the iris to bulge anteriorly ("iris bombé").
This bulging of the iris is maximal when the pupil is in the middilated
position, generally 3 to 6 mm in diameter. The major factor that seems to
predispose to an acute attack of ACG is prolonged, stationary middilation
of the pupil. This sustained middilation can be seen with prolonged awake
exposure to dim light or darkness with relaxation of the sphincter
muscle, topical or systemic pharmacologic therapy that dilates the pupil,
or prolonged severe emotional stress with secondary dilation due to
adrenergic stimulation of the dilator muscle.
ACG presents as an acutely
inflamed eye. Eye pain or headache varies in severity. Nausea and
vomiting are common and may be the presenting complaints. As the IOP
reaches the range of 50 to 60 mmHg, fluid is forced into the normal
cornea, resulting in corneal edema. Because of this, patients may report
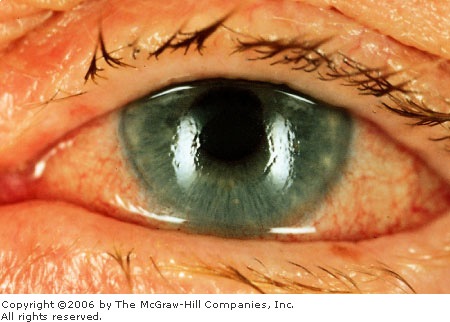
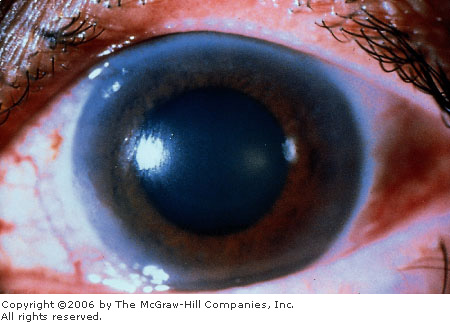
blurred vision and rainbow-colored halos around lights. Clinical findings
on examination include tearing, conjunctival injection with a perilimbal
("ciliary") flush, a cloudy ("steamy") cornea (Fig.
2.21), a nonreactive and middilated pupil (Fig. 2.22), mild anterior
chamber inflammation, and increased intraocular pressure. Also, the
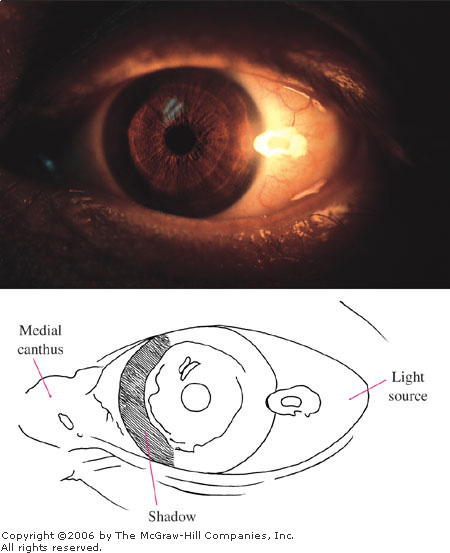
anterior chamber is shallow. This is demonstrated by a penlight held
laterally and directed nasally (Fig. 2.23). In an eye with a normal
anterior chamber, the entire iris will be illuminated by the penlight. In
an eye with a narrow angle or shallow anterior chamber, a shadow is cast
on the nasal side of the iris secondary to the forward bowing of the
iris. Alternatively, slit-lamp biomicroscopy may also be used to assess
the anterior chamber's depth. Funduscopic examination demonstrates optic
cupping only if there is preexisting glaucoma.
|
|
|
|
|
Acute
Angle Closure Glaucoma The
cornea is edematous, manifest by the indistinctness of the iris
markings and the irregular corneal light reflex. Conjunctival
hyperemia is also present. (Courtesy of Kevin J. Knoop, MD, MS.)
|
|
|
|
|
|
|
Acute
Angle Closure Glaucoma Note
the cloudy or "steamy" appearance of the cornea and the
midposition pupil. Conjunctival hyperemia is not as evident.
(Courtesy of Gary Tanner, MD.)
|
|
|
|
|
|
|
Penlight
Test A penlight, held
laterally and directed nasally, projects a shadow on the nasal side
of an iris with a shallow anterior chamber. This patient presented
with acute angle closure glaucoma. (Courtesy of Alan B. Storrow, MD.)
|
|
Tonometers available in the ED
for measurement of IOP include the air-puff noncontact tonometer, the
Schiøtz tonometer, the Tono-Pen, and the applanation tonometer. Tactile
tonometry, using the examiner's fingers to ballot the globe, can easily
detect ACG in patients with markedly elevated IOP.
Differential Diagnosis
Similar conditions include
temporal arteritis, acute iritis, ulcer, and other causes of a red eye
(conjunctivitis, trauma, scleritis, keratitis, foreign body).
Emergency Department Treatment
and Disposition
ACG requires emergent
ophthalmology consultation. Treatment is directed at reducing the IOP.
Aqueous outflow is increased by the use of topical miotics (pilocarpine 1
or 2%, one drop every 5 min x 2. If
the pupil does not respond, one drop every hour x 4 should be administered.) Topical miotics such as
pilocarpine stimulate miosis to pull the peripheral iris taut, away from
the trabecular meshwork. If the pupil does not respond within 5 to 10 min
(or if it is not expected to respond because the IOP is greater than 50
to 60 mmHg or the attack is many hours old), other medications with a
different mechanism of action to decrease the IOP should be administered.
Decreased production of aqueous is accomplished with the use of a topical
beta blocker (timolol maleate, 0.5%, one drop every 12 h) or an
alpha-adrenergic agonist (apraclonidine, 1%, one drop every 12 h).
Acetazolamide, a carbonic anhydrase inhibitor, can also be given (500 mg
PO or 500 mg IV if the patient is nauseated or vomiting). The goal of
these medicines, which decrease the production of aqueous, is to rapidly
lower the IOP to less than 40 to 50 mmHg so as to allow for reperfusion
of the pupillary sphincter, thereby permitting the muscle to respond to
pilocarpine. Osmotic agents may also be used. Oral agents are tried
first; if they are unsuccessful or if the patient is nauseous, systemic
intravenous agents may then be used. Oral agents include glycerol (1.0 to
1.5 g/kg in a 50% solution). In diabetics, since glycerol can cause
hyperglycemia, oral isosorbide can be substituted (1.5 to 2.0 g/kg).
Intravenous agents include mannitol (20% solution, 2 g/kg given over 30
min). Hyperosmotics realize their maximal reduction within 45 to 60 min.
A final agent to consider for use in ACG is the prostaglandin derivative
latanoprost (0.005%, one drop once daily in the evening). Its mechanism
of action is increased uveal-scleral outflow, acting to increase aqueous
outflow through nontrabecular meshwork pathways.
Corneal indentation may be used
in situations where the IOP is 50 mmHg or greater and topical miotics are
ineffective secondary to ischemia of the iris constrictor. Indentation of
the cornea displaces the aqueous to the peripheral anterior chamber,
temporarily opening the angle. Corneal indentation is performed with
topical anesthetics and any smooth instrument such as the Goldmann
applanation prism. The prism is held with the fingers and firm pressure
is applied for 30 s. This may successfully decrease the IOP and abort the
attack; if the IOP has been of long standing, however, it is more likely
to be unsuccessful.
The definitive treatment of ACG
is laser peripheral iridectomy, usually done after the IOP normalizes.
Clinical Pearls
1. ACG may be inadvertently
precipitated in the ED patient treated for corneal abrasion with a
cycloplegic (cyclopentolate). Therefore the depth of the anterior chamber
should always be evaluated in ED patients receiving cycloplegics.
2. In light of the associated
severe headache and vomiting, patients with ACG may easily be
misdiagnosed as having a migraine headache or a central nervous system
(CNS) catastrophe.
3. The unaffected eye will also
have a narrow anterior chamber. The presence of a shallow anterior
chamber in only one eye casts doubt on the diagnosis of ACG, since ACG is
the result of an anatomic configuration that is almost always bilateral.
4. Patients with ACG may be
able to recall a previous (milder) attack that they felt was a migraine
or some other type of headache.
5. The elevated IOP in acute
ACG can be reduced pharmacologically by three mechanisms: first, by
opening the closed angle with miotics; second, by reducing aqueous
formation with beta blockers, alpha agonists, and carbonic anhydrase
inhibitors; and third, by reducing the aqueous volume within the eye
using osmotic agents.
|
|
Anterior Uveitis (Iritis)
Associated Clinical Features
The uvea is the middle layer of
the eye and is composed of the iris, ciliary body, and choroid. Uveitis
refers to inflammation within the uvea, and anterior uveitis localizes
the inflammation to the anterior chamber, iris, ciliary body, and
anterior vitreous.
In many cases of anterior
uveitis, no definitive diagnosis can be made. A significant number,
however, are associated with medically treatable systemic diseases. The
list is extensive and includes inflammatory disorders (juvenile
rheumatoid arthritis, rheumatoid arthritis, sarcoidosis, Behçet's
disease, Sjögren's syndrome), conditions associated with HLA-B27,
(ankylosing spondylitis, inflammatory bowel disease, Reiter's syndrome),
and infectious diseases (tuberculosis, toxoplasmosis, herpes simplex,
herpes zoster, cytomegalovirus, syphilis, AIDS). Finally, lymphoma and
Kawasaki's disease may also manifest as an anterior uveitis. Therefore
aggressive attempts to determine the underlying cause of the uveitis are
warranted.
Clinical features of anterior
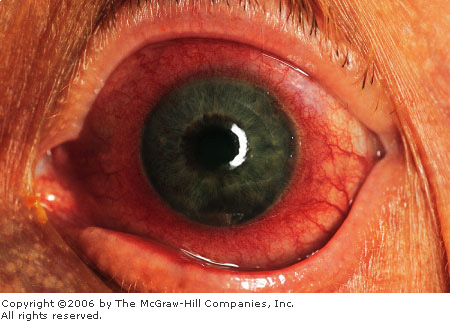
uveitis include conjunctival hyperemia, hyperemic perilimbal vessels
("ciliary flush") (Fig. 2.24), decreased visual acuity,
photophobia, miosis, pupillary irregularities (secondary to the formation
of anterior or posterior synechiae), iris nodules, tearing (particularly
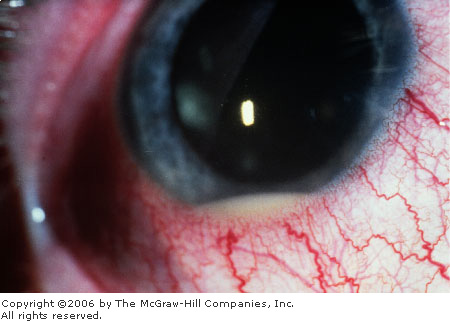
when exposed to bright lights), and pain. A hypopyon (a layer of white
blood cells in the dependent portion of the anterior chamber) may be seen
(Fig. 2.25). The slit-lamp examination may demonstrate cells and flare. Cells
refers to the finding of inflammatory cells seen in the anterior chamber,
having the appearance of dust in a sunbeam (Fig. 2.26); flare is
light scatter secondary to inflammatory cells and proteins circulating
within the aqueous and anterior chamber and has the appearance of a
headlight in fog (Fig. 2.27). Other significant slit-lamp findings in
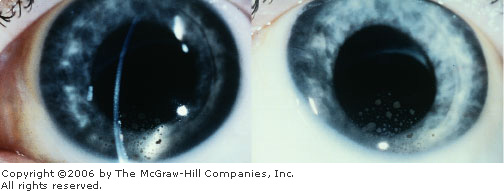
anterior uveitis include keratic precipitates, which are agglutinated
inflammatory cells adherent to the posterior corneal endothelium (Fig.
2.28). Keratic precipitates appear either as fine gray-white deposits or
as large, flat, confluent areas with a greasy surface ("mutton
fat"). The intraocular pressure (IOP) may be elevated secondary to
inflammatory debris within the trabeculae, obstructing outflow, or the
IOP may be decreased secondary to decreased production of aqueous humor
by the inflamed ciliary body.
|
|
|
|
|
Anterior
Uveitis Marked conjunctival
injection and perilimbal hyperemia ("ciliary flush") are seen
in this patient with recurrent iritis. (Courtesy of Kevin J. Knoop,
MD, MS.)
|
|
|
|
|
|
|
Hypopyon A thin layering of white blood cells is
present in the inferior anterior chamber. (Used with permission from
Spalton DJ, Hitchings RA, Hunter PA (eds): Atlas of Clinical
Ophthalmology, 2d ed. Mosby-Wolfe Limited, London, UK, 1994.)
|
|
|
|
|

|
|
Anterior
Chamber Cells Cells in the
anterior chamber are a sign of inflammation or bleeding and appear
similar to particles of dust in a sunbeam. They are best seen with a
narrow slit-lamp beam directed obliquely across the anterior chamber.
(Used with permission from Spalton DJ, Hitchings RA, Hunter PA (eds):
Atlas of Clinical Ophthalmology, 2nd ed. Mosby-Wolfe Limited,
London, UK, 1994.)
|
|
|
|
|
|
|
Anterior
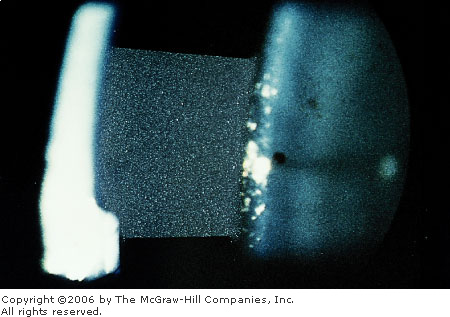
Chamber Flare Flare in the
anterior chamber represents an elevated concentration of plasma
proteins from inflamed, leaking intraocular blood vessels. Flare seen
in a slit-lamp beam appears similar to a car headlight cutting
through the fog. (Used with permission from Spalton DJ, Hitchings RA,
Hunter PA (eds). Atlas of Clinical Ophthalmology. Mosby-Wolfe
Limited, London, UK, 1984.)
|
|
|
|
|
|
|
Keratic
Precipitates Deposits of cells
on the endothelial layer of the cornea are seen in these
photographs. (Used with permission from Spalton DJ, Hitchings RA,
Hunter PA (eds): Atlas of Clinical Ophthalmology, 2nd ed.
Mosby-Wolfe Limited, London, UK, 1994.)
|
|
Differential Diagnosis
Other conditions presenting with
a red eye include glaucoma, conjunctivitis (bacterial, viral, allergic),
scleritis, episcleritis, keratitis, and corneal ulcer. A hypopyon can
also be seen with severe corneal ulcerations and penetrating trauma to
the anterior chamber.
Emergency Department Treatment
and Disposition
In light of the common
association of anterior uveitis with systemic disease, the evaluation of
a uveitis patient in the ED is a systemic evaluation. The history should
focus on rheumatic illness, dermatologic problems, bowel disease,
infectious exposures, and sexual history. This history forms the basis
for the subsequent physical examination and laboratory testing.
Treatment of the anterior uveitis
itself is nonspecific. Topical cycloplegics (atropine) and
corticosteroids may be prescribed in conjunction with the
ophthalmologist. Although nonspecific, this therapy will greatly reduce
the amount of scarring. Antibiotics are not usually prescribed or helpful
unless there is a bacterial origin. Prompt ophthalmology follow-up is
important if steroids are prescribed.
Clinical Pearls
1. Key diagnostic features of
anterior uveitis are a miotic pupil, ciliary flush, and the finding of
cells and flare in the anterior chamber.
2. When cells and flare are
visualized in the anterior chamber using the slit lamp, the cells look
like dust particles in a sunbeam, and the flare (the slit-lamp beam)
looks like a headlight cutting through fog.
3. Inflammation of the anterior
uveal tract (iris or ciliary body) produces the clinical symptom of
photophobia.
4. The presence of anterior
uveitis requires a search for associated systemic illness.
5. Topical analgesics
(tetracaine, proparacaine) do not significantly ameliorate the pain of
anterior uveitis, unlike the case with many of the common conditions seen
in the ED, such as corneal abrasions.
6. The discharge associated
with anterior uveitis is watery or nonexistent. A purulent discharge
suggests an infectious condition such as conjunctivitis or keratitis.
|
|
Herpes Zoster Ophthalmicus
Associated Clinical Features
Herpes zoster ophthalmicus
develops secondary to activation of latent varicella zoster virus within
the trigeminal ganglion. Neuronal spread of the virus through the
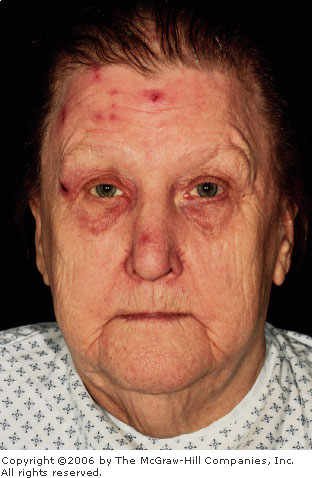
ophthalmic division of the trigeminal nerve results in crops of grouped
vesicles in a dermatomal distribution (Fig. 2.29).
|
|
|
|
|
Herpes
Zoster Ophthalmicus A healing
vesicular rash in the distribution of the ophthalmic division (V1) of
the trigeminal nerve is present in this 72-year-old diabetic patient.
The presence of the lesion on the tip of the nose (Hutchinson's sign)
increases the risk of ocular involvement. (Courtesy of Frank Birinyi,
MD.)
|
|
Almost any ophthalmic abnormality
in both the anterior and posterior segments of the eye may be seen with
herpes zoster ophthalmicus. The most common corneal lesion is punctate
epithelial keratitis, in which the cornea has a ground-glass appearance
because of stromal edema. Pseudodendrites are also very common.
Pseudodendrites form from the deposition of mucus, are usually
peripherally located and stain moderately to poorly with fluorescein.
Pseudodendrites may be differentiated from the dendrites of herpes
simplex infection in that the pseudodendrites lack the rounded terminal
bulbs at the end of the branches and are broader and more plaque-like.
When they are wiped from the cornea, a layer of intact epithelium may
remain, unlike the full-thickness epithelial defect seen with herpes
simplex. The third most common corneal lesion—after punctate
epithelial keratitis and pseudodendrites—is that of anterior
stromal infiltrates. These are seen between the second and third weeks
after the acute disease and are felt to be an immune response to viral
antigen diffusing into the anterior stroma. They may be single or
multiple.
Corneal anesthesia or
hypoesthesia is a frequent complication of herpes zoster keratitis. Some
60% of patients will recover essentially normal sensitivity within 2 to 3
months. In about 25%, however, the anesthesia is permanent.
The anterior uvea is commonly
involved in herpes zoster ophthalmicus and is second only to the cornea
in frequency of involvement. Anterior uveitis may develop early or years
after the acute disease—and independent of corneal pathology.
Clinical findings range in severity and include ciliary flush, miosis,
pain, cells and flare in the anterior chamber, photophobia, visual
decrease, keratic precipitates (agglutinated inflammatory cells adherent
to the posterior corneal endothelium), and anterior and posterior
synechiae (adhesions from the iris to the cornea anteriorly or to the
lens posteriorly).
Conjunctivitis is also extremely
common; it is characterized by a watery discharge. Follicles (hyperplastic
lymphoid tissue that appears as gray or white lobular elevations,
particularly in the inferior cul-de-sac) and regional adenopathy may or
may not be present.
Differential Diagnosis
Keratitis secondary to herpes
simplex may also present with rash, red eye, and dendriform corneal
lesions. Ocular complications of herpes zoster ophthalmicus may follow
the rash by many months to years; they have a highly variable
presentation that can mimic almost any ophthalmic disease.
Emergency Department Treatment
and Disposition
In patients with epithelial
defects, topical broad-spectrum antibiotics should be administered bid to
prevent secondary infection. Oral valacyclovir (1 g three times a day for
7 days), famciclovir (500 mg three times a day for 7 days), or acyclovir
(800 mg five times a day for 10 days) is beneficial, particularly if
given within 72 h of onset. Topical antivirals are clinically
ineffective. Cycloplegics (cyclopentolate) are used if an iritis is
present. Artificial tears and ointment may be recommended to the patient
as necessary. Nonnarcotic and narcotic analgesics may be prescribed. An
ophthalmology consult is appropriate.
Steroids in the past (before
acyclovir) were among the mainstays of treatment. However, steroids have
proved less successful than systemic acyclovir, famciclovir, and
valacyclovir. Also arguing against steroid use is the risk of systemic
dissemination of the herpes zoster virus should the zoster be an early
presenting sign of immunosuppression. Therefore systemic steroids are
largely out of favor.
Clinical Pearls
1. Nearly two-thirds of
patients with herpes zoster ophthalmicus develop ocular lesions; thus
careful eye examination with corneal staining should be performed to rule
out corneal involvement.
2. Patients with skin lesions
on the tip of the nose (Hutchinson's sign) are at higher risk for ocular
involvement with herpes zoster. The sensory innervation to both the eye
and the tip of the nose is supplied by the nasociliary branch of the
ophthalmic division of cranial nerve V. This sign, however, is highly
variable in its reliability. The eye may be involved without nasal
involvement.
3. Corneal hypoesthesia and the
appearance of dendrites with fluorescein staining are seen in both herpes
zoster ophthalmicus and herpes simplex keratitis.
4. The severity of the
cutaneous disease does not necessarily correlate with the severity of the
ocular disease.
5. The prognosis is good, and
recurrences—unlike those for herpes simplex keratitis—are
rare.
|
|
Ocular Herpes Simplex
Associated Clinical Features
Ocular herpetic disease may be
neonatal, primary, or recurrent. Neonatal ocular herpes develops
secondary to passage through an infected birth canal. Usually (80%) is
herpes simplex virus (HSV) type 2. Most frequently the infection is a
conjunctivitis, often associated with a keratitis. Fetal monitoring with
a scalp electrode is associated with an increased risk for neonatal HSV
infection.
Primary ocular herpes is an acute
first HSV infection of a nonimmune host. It may present as blepharitis
(vesicles on an erythematous base), conjunctivitis, or
keratoconjunctivitis. Clinical disease may be seen 3 to 9 days after
exposure. Patients with keratoconjunctivitis commonly have significant
periorbital skin involvement. They note pain, irritation, foreign-body
sensation, redness, photophobia, tearing, and occasionally decreased
visual acuity. Follicles and preauricular adenopathy may be seen.
Initially the keratitis is diffuse and punctate. After 24 h, fluorescein
stain appears as either serpiginous ulcers without clear-cut branching or
multiple diffuse microdendritic epithelial defects. True dendritic ulcers
are rarely seen in primary disease.
The most likely clinical scenario
facing the ED physician in cases of ocular herpes simplex is that of
recurrent disease. Recurrences may be triggered by immunosuppression,
fever, ultraviolet light exposure, trauma, systemic illness, stress, or
menstruation. In recurrent disease, keratoconjunctivitis (Fig. 2.30),
blepharitis, or iritis is seen, and the cornea is more likely to be
involved (Fig. 2.31). With blepharitis and recurrent HSV disease,
vesicles are grouped in focal clusters, with significantly less skin
involvement than that seen in primary herpes. In those patients with
recurrent HSV and keratoconjunctivitis, signs and symptoms include a
watery discharge, conjunctival injection, irritation, blurred vision, and
preauricular lymph node involvement. Corneal involvement initially may
appear punctate but evolves into a characteristic dendritic keratitis
(Figs. 2.32, 2.33). Dendritic ulcers may be single or multiple. Their
linear branches classically end in bead-like extensions called terminal
bulbs. These are best seen with rose Bengal stain, which stains the
epithelial defect as well as the infected cells surrounding the defect
(Fig. 2.34). Fluorescein dye demonstrates primarily the corneal defect;
punctate staining may be appreciated over the surrounding damaged
epithelium. In addition to the dendritic pattern, fluorescein staining
may instead take on a geographic appearance. This is particularly true
when topical steroids have been (incorrectly) prescribed. Most patients
(80%) with herpes simplex keratitis have decreased or absent corneal
sensation in the area of the dendrite or geographic ulceration.
Uninvolved areas of the cornea may retain normal sensation.
|
|
|
|
|
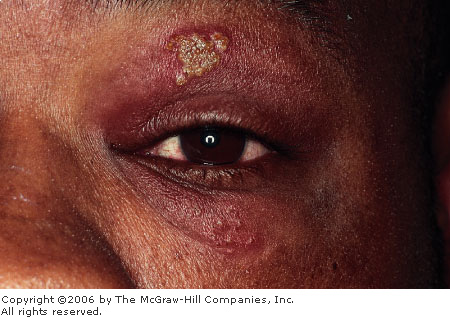
Herpes
Simplex Keratitis This
23-year-old has a history of ocular herpes infections since
childhood. Grouped vesicles on an erythematous base with mild lid
swelling are seen. The conjunctiva is mildly hyperemic. (Secondary
impetigo may be present.) (Courtesy of Frank Birinyi, MD.)
|
|
|
|
|
|
|
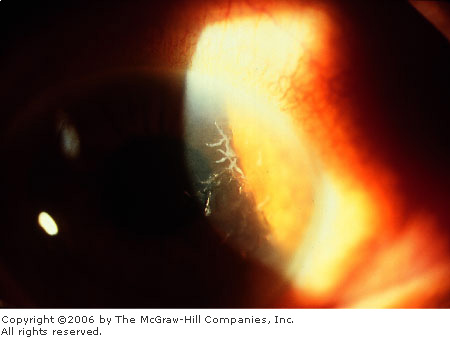
Herpes
Simplex Keratitis A slit-lamp
view of unstained dendritic lesions. (Courtesy of Lawrence B. Stack,
MD.)
|
|
|
|
|
|
|
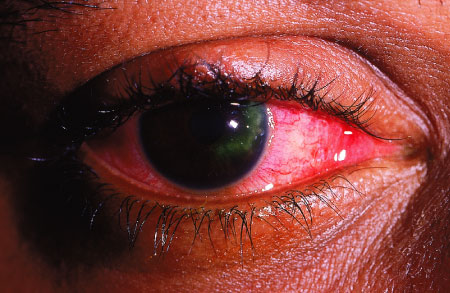
Herpes
Simplex Keratitis A large
dendritic lesion after fluorescein staining. The patient had been
diagnosed with "pink eye" in a prior visit. (Courtesy of
Kevin J. Knoop, MD, MS.)
|
|
|
|
|

|
|
Herpes
Simplex Keratitis A magnified
view via slit-lamp biomicroscopy shows the classic terminal bulbs
pathognomonic for ocular HSV infection. (Courtesy of Department of
Ophthalmology, Naval Medical Center, Portsmouth, VA.)
|
|
|
|
|

|
|
Herpes
Simplex Keratitis Fluorescein
(left) and rose Bengal (right) stains demonstrate
characteristic dendritic patterns. Whereas fluorescein staining is
used to detect epithelial defects, rose Bengal staining additionally
demonstrates degenerating or dead epithelial cells and is
particularly good for demonstrating the club-shaped terminal bulbs at
the end of each branch. (Used with permission from the American
Academy of Ophthalmology: External Disease and Cornea: A
Multimedia Collection, San Francisco, 1994.)
|
|
Following protracted and repeated
episodes of HSV keratitis, corneal lesions occasionally scar, and a
permanently decreased visual acuity can result. In some patients, melting
and perforation of the cornea ensue secondary to structural damage.
Differential Diagnosis
Other causes of red eye
(scleritis, iritis, glaucoma, conjunctivitis) should be considered. A
similar fluorescein appearance may be seen with herpes zoster virus, recurrent
corneal erosions, or a healing corneal abrasion.
Emergency Department Treatment
and Disposition
In neonatal ocular herpes
infections, an emergency pediatric or infectious disease consultation is
initiated. There is a high association between ocular neonatal HSV
infection and potentially lethal systemic or neurologic infection.
Therefore acyclovir is given intravenously. The ocular disease itself may
be treated with topical antivirals (idoxuridine, vidarabine,
trifluorothymidine).
Treatment of patients (beyond the
neonatal period) with primary ocular herpes is usually successful and
generally results in healing without scarring. For those with blepharitis
or periocular dermatitis, warm wet soaks bid and general good hygiene is
recommended. A prophylactic topical antiviral (trifluorothymidine drops
five times a day, idoxuridine ointment tid or idoxuridine drops eight
times a day) is administered to the adjacent eye until the skin lesions
scab and dry. Patients with corneal involvement require a 2- to 3-week
course of antiviral ointment or drops (trifluorothymidine 1% nine times a
day, or idoxuridine 0.1% either q 1 h by day, q 2 h at night, or 1 drop q
5 min x 5 five times daily). Topical
antibiotics bid are also recommended for those with corneal lesions to
prevent secondary bacterial infection.
In patients with recurrent
disease limited to HSV blepharitis, the lesions are superficial and heal
without scarring. The goal of therapy in these patients is to protect the
globe with prophylactic antiviral ointment tid or drops five to six times
a day until the lesions have scabbed. No clinical trials have
demonstrated oral acyclovir to prevent herpetic blepharitis. Nevertheless
initiating acyclovir 400 mg PO five times a day for 5 days within 1 h of
the first sign of recurrence is recommended and may alleviate some
symptoms.
For most patients with recurrent
HSV disease and corneal involvement, topical antivirals alone are
effective. Trifluorothymidine (1% nine times a day for 14 to 21 days) is
recommended. Topical antibiotics are administered bid while a corneal
defect is present. Ophthalmology consultation is required.
Clinical Pearls
1. The diagnosis of acute
neonatal ocular HSV should be entertained in any infant with nonpurulent
conjunctivitis or keratitis.
2. HSV is the most common cause
of corneal ulceration and the most common infectious cause of corneal
blindness in the western hemisphere.
3. HSV dendrites, when stained
with fluorescein, appear as branching lesions with club-shaped or
bead-like extensions called terminal bulbs at the end of each branch. In
primary HSV disease, however, dendrites are rare. Terminal bulbs are not
seen in herpes zoster dendrites.
4. Other patterns of
fluorescein staining in HSV infection include a superficial punctate
keratitis. This is seen particularly in primary disease and early in the
course of recurrent disease.
5. With recurrent attacks,
corneal pain may be diminished owing to increasing corneal hypoesthesia.
|
|
Corneal Ulcer
Associated Clinical Features
The term corneal ulcer
denotes an inflammatory and ulcerative condition. Similar terms include infectious
keratitis, bacterial keratitis, and ulcerative keratitis.
Etiologies include bacteria (most commonly Staphylococcus,
Streptococcus, and Pseudomonas) and viruses (herpes simplex).
(Keratitis secondary to herpes simplex is discussed separately.)
Bacterial corneal ulcers are commonly associated with extended-wear soft
contact lenses and contaminated lens care solutions (Fig. 2.35), whereby
minor corneal abrasion or chemical damage permits bacteria to penetrate
the cornea. Fungal infection, although rare, should be suspected in cases
of ocular trauma involving vegetable matter (a tree branch), chronic
corneal disease (herpes keratitis), or cases involving steroid use. Acanthamoeba
keratitis is associated with contaminated contact lens solutions.
|
|
|

|
|
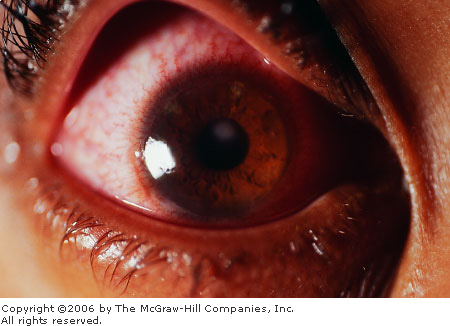
Corneal
Ulcer An elliptical ulcer at 5
o'clock near the periphery is seen. This location is atypical for a
bacterial ulcer. The patient presented with painful red eyes and
normal uncorrected vision, but was a new wearer of soft contact
lenses (for cosmesis). Bilateral corneal ulcers were diagnosed, which
cleared after treatment with topical cipro-floxacin. The impressive
ciliary flush is pathognomonic for corneal (versus conjunctival)
pathology. (Courtesy of Kevin J. Knoop, MD, MS.)
|
|
Symptoms associated with corneal ulcer include pain,
photophobia, decreased vision, discharge, and a foreign-body sensation.
The ulcer appears as a corneal stromal infiltrate associated with
conjunctival hyperemia (Fig. 2.36), a miotic pupil, and chemosis along
with lid edema and erythema. Slit-lamp biomicroscopy demonstrates an
epithelial defect with fluorescein uptake. Findings in the anterior
chamber include cells (inflammatory cells that look like dust in a
sunbeam) and flare (light scatter seen secondary to cells and proteins),
keratic precipitates (inflammatory cells that have coalesced and adhered
to the cornea), and hypopyon (a layer of white blood cells in the
inferior or dependent portion of the anterior chamber).
|
|
|
|
|
Corneal
Ulcer A circular corneal
infiltrate is seen at 12 o'clock; conjunctival hyperemia is present.
(A rectangular flash reflection is seen at 9 o'clock.) A mild limbal
flush is noted superiorly. (Courtesy of Lawrence B. Stack, MD.)
|
|
Differential Diagnosis
Other possibilities include a
residual corneal foreign body, a rust ring, and a sterile corneal
infiltrate (secondary to an immune reaction to contact lens solution or Staphylococcus).
Other causes of a red eye (conjunctivitis, glaucoma, scleritis) should
also be considered.
Emergency Department Treatment
and Disposition
Corneal ulcers should be treated
as an ophthalmologic emergency and assumed to be bacterial until proven
otherwise. An emergent ophthalmology consult is indicated, and stains and
cultures should be obtained as expeditiously as possible before
antibiotic treatment has commenced. Intensive topical treatment is the
most effective way to treat corneal infections. Fortified (concentrated)
cephalosporins or vancomycin combined with an aminoglycoside may be used
every 30 to 60 min. A fluoroquinolone may be used as a single agent for
mild cases of infectious keratitis. Clinical improvement is usually noted
after 2 to 3 days of therapy; the frequency of antibiotic instillation
can then be tapered. Subconjunctival injections of antibiotics every 12
to 24 h are used in severe cases. Systemic antibiotics are not usually
used except in cases where the organism may have extended to the sclera (Pseudomonas)
or if there is a high risk of concurrent systemic disease (Neisseria,
Haemophilus). Cycloplegics (atropine) are usually recommended if
there is an accompanying iritis. Epithelial debridement may be
beneficial. Steroids and an eye patch are contraindicated in the initial
management. A contact lens wearer must discontinue contact lens wear.
Clinical Pearls
1. A bacterial corneal
infection must be treated as an ophthalmologic emergency.
2. Corneal injury, including
recent contact lens procedures, is a risk factor for the development of a
corneal ulcer.
3. Pseudomonas aeruginosa
is capable of destroying the cornea within 6 to 12 h. It should be
suspected by its aggressive course, thick yellow-green or blue-green
tenacious, mucopurulent exudate, and ground-glass edema surrounding the
ulcer.
4. Acanthamoeba, a
ubiquitous protozoan, should be suspected in contact lens wearers with
contaminated lens solutions or who swim wearing their contact lenses.
These patients characteristically have pain out of proportion to their
clinical findings.
5. Infectious ulcers tend to
develop centrally, away from the vascular supply and immune system of the
limbus, but they can also be peripheral.
|
|
Anisocoria
Associated Clinical Features
Anisocoria is unequal pupil size.
In room lighting, the normal diameter of pupils is 3 to 5 mm. Pupil size
is larger in childhood and smaller with age. Anisocoria of 1 to 2 mm may
be a normal finding, and is found in 5 to 20% of normal individuals. With
normal (physiologic) anisocoria, the disparity in pupil size is the same
in light as in dark. In addition, the pupils react normally to light and
accommodation; they are perfectly round, extraocular movements are
intact, and no ptosis or conjunctival injection is present.
Differential Diagnosis
Pupillary inequality is
indicative of damage to one of the four iris muscles or their
innervation. In order to establish which is the abnormal pupil, pupil
sizes in light and dark should be compared. Anisocoria increases in the
direction of action of the paretic iris muscle. If the iris sphincter
muscle is paretic, its weakness will be accentuated in bright light,
since reflex constriction is impaired. Conversely, if the iris dilator
muscle is paretic, the anisocoria will be accentuated in darkness.
The most serious causes of
anisocoria are neurogenic. In the setting of trauma, severe headache, or
following intracranial surgery, anisocoria and a third-nerve palsy with
ptosis, extraocular muscle palsies, and a nonreactive pupil are early
signs of an aneurysm or an expanding supratentorial mass with tentorial
herniation.
Other causes of anisocoria with
an abnormally large pupil include an acute Adie's pupil, eye drops
(atropine, phenylephrine, naphazoline), inadvertent contamination from a
scopolamine patch, and ocular trauma with damage to the iris sphincter
(Fig. 2.37). A patient with an Adie's pupil may note that he or she
cannot focus with one eye, although the appearance of a dilated pupil is
more likely to prompt the ED visit. An Adie's pupil is dilated secondary
to impairment in the postganglionic nerve supply to the iris sphincter.
Causes include orbital trauma, infection, herpes zoster, diabetes,
autonomic neuropathies, and Guillain-Barré syndrome, but in most
instances the cause is unknown. Although an Adie's pupil is initially
dilated, it may become miotic over time. A dilated pupil that is
secondary to atropine eye drops does not react to light. Sympathomimetics
eye drops such as phenylephrine, on the other hand, dilate the pupil, but
the pupil still has some response to light. In addition, the conjunctiva
may be blanched and the lid retracted. The slit-lamp examination helps to
differentiate drug-induced dilation from dilation secondary to Adie's
pupil. When dilation is drug-induced, the entire sphincter muscle is
affected, whereas in Adie's, a segmental palsy is seen. (Segmental palsy
is best appreciated with slit-lamp biomicroscopy. The curvature of the
pupillary margin in those sections of the iris with palsy appears
flatter. In addition, active pupil constriction is not radially
symmetric.) The patient with ocular trauma and damage to the iris
sphincter may show, on slit-lamp examination, evidence of a torn iris, an
irregularly shaped pupil, hyphema, or lens dislocation.
|
|
|
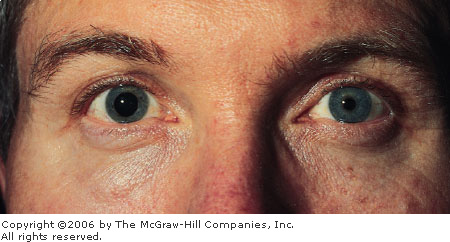
|
|
Normal
Anisocoria Marked chronic
anisocoria secondary to prior trauma. (Courtesy of Kevin J. Knoop,
MD, MS.)
|
|
An abnormally small pupil may be
secondary to Horner's syndrome (see Fig. 2.38), chronic Adie's pupil (8
weeks or more after the event), iritis, and eye drops (pilocarpine).
Associated clinical findings in Horner's syndrome include mild to
moderate ptosis. The patient with iritis usually demonstrates
conjunctival injection with a ciliary flush. Unilateral miosis may be
transiently observed after ocular injury, followed by the development of
mydriasis. Miosis in the setting of ocular trauma is secondary to spasm
of the pupillary sphincter muscle.
Emergency Department Treatment
and Disposition
The evaluation of anisocoria in
the ED is dependent on the clinical presentation. A patient with acute
onset of a third-nerve palsy and associated headache or trauma should be
aggressively evaluated and treated as a neurosurgical emergency.
Pilocarpine may be helpful to
differentiate pharmacologic pupil dilation from other causes of an
abnormally dilated pupil, such as Adie's pupil and third-nerve palsy.
With low concentrations of pilocarpine (0.125%), an Adie's pupil will
constrict significantly more than the unaffected pupil because of
denervation supersensitivity. With higher concentrations (1%), a pupil
will constrict even if there is a third-nerve palsy. A pupil that fails
to constrict with 1% pilocarpine localizes the etiology to the iris
sphincter muscle itself. In this situation, the most likely diagnosis is
the use of topical anticholinergic mydriatics such as scopolamine,
atropine, or cyclopentolate. Other problems within the iris sphincter
muscle that prevent constriction to 1% pilocarpine include synechiae
(causing a mechanically immobile iris) and trauma to the iris muscle.
Clinical Pearls
1. The patient who presents
with a dilated pupil should undergo a careful examination for ptosis and
abnormal extraocular muscle movements, since their presence suggests
compressive damage to the intracranial third nerve by an aneurysm or
mass.
2. Pupil sizes in light and
dark should be compared. In physiologic or normal anisocoria, the
disparity in pupil size is the same in light as in dark. Anisocoria that
is greater in the dark suggests that the smaller pupil is abnormal due to
paresis of its iris dilator muscle; anisocoria greater in the light
suggests that the larger pupil is abnormal due to paresis of its iris
sphincter muscle.
3. An old photograph or
driver's license viewed with an ophthalmoscope may be helpful to document
the prior existence of anisocoria.
4. Some brands of eye makeup
contain belladonna alkaloids, which can cause mydriasis.
5. Adie's pupil initially is
dilated but may become miotic over time. It is a benign condition that
affects young adults, women more often than men.
|
|
Horner's Syndrome
Associated Clinical Features
Horner's syndrome consists of
loss of ocular sympathetic innervation secondary to a lesion within the
sympathetic pathway. This pathway can be interrupted in any location,
from the hypothalamus down through the brainstem to the cervical cord, in
the apex of the chest, along the carotid sheath, or in the cavernous
sinus or orbit. Clinical features include unilateral miosis, along with
mild to moderate unilateral ptosis (Fig. 2.38). Slight elevation of the
lower lid may also be noted. Light and near reactions are intact.
Acutely, conjunctival injection may be present on the affected side, and
the intraocular pressure may be reduced. Anhidrosis of the ipsilateral
face may be present, and the ipsilateral face may be warm and hyperemic.
However, these findings are not present if the interruption of the
sympathetic nerve supply occurs in a distal location, such as along the
internal carotid artery. This is because vasoconstrictor fibers to the
face, along with innervation to sweat glands, travel with branches of the
external carotid artery.
|
|
|
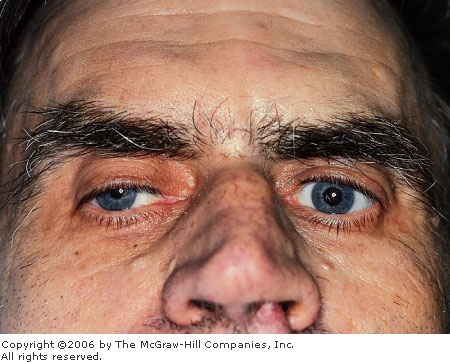
|
|
Horner's
Syndrome Unilateral miosis and
ptosis are seen in this patient with Horner's syndrome and sarcoma
metastatic to the spine. (Courtesy of Frank Birinyi, MD.)
|
|
A pupillary finding that is
specific to Horner's syndrome is dilation lag. Normal pupil dilation is a
combination of sphincter relaxation and dilator contraction, which
produces a prompt dilation. A patient with Horner's syndrome, however,
has a weak dilator muscle in one iris; as a result, that pupil dilates
more slowly than the normal pupil. This anisocoria is maximal after 4 to
5 s. After 10 to 20 s, the anisocoria lessens, owing to the continued
relaxation of the iris sphincter. Dilation lag is best seen if the
patient is examined in a room where the lights can be turned off and a
hand-held light illuminates the eyes from below.
Associated clinical findings are
secondary to the neurologic lesion and its location. The causes of
Horner's syndrome may involve first-order neurons (stroke or tumor in the
hypothalamus and brainstem), second-order neurons (cervical spinal
trauma, syringomyelia, cervical chain involvement by a Pancoast tumor,
cervical rib, or tumor) or third-order neurons (internal carotid artery
dissection, craniofacial or orbital trauma). Second-order neuron
involvement may also occur during inadvertent injury from the placement
of a central venous line or chest tube.
Horner's syndrome secondary to
involvement of third-order neurons is often idiopathic.
Differential Diagnosis
Ptosis is seen in third-nerve
palsies. Miosis may be due to eye drops (pilocarpine), iritis, an
Argyll-Robertson pupil (a syphilitic pupil that accommodates but does not
react), and long-standing Adie's pupil.
Pupillary inequality may be due
to physiologic anisocoria.
Emergency Department Treatment
and Disposition
Horner's syndrome is often caused
by vascular disease, trauma, or tumor. The ED physician should attempt to
elucidate associated signs and symptoms that help to localize the lesion.
A patient with Horner's syndrome and cranial nerve abnormalities likely
has brainstem or intracavernous pathology. Therefore an associated
ipsilateral abducens palsy suggests a cavernous sinus lesion, since
ocular sympathetics travel with the abducens nerve within the cavernous
sinus. Wallenberg's syndrome, infarction of the lateral medulla, should
be considered in a patient with numbness in the ipsilateral face and
contralateral extremities. These types of patients should undergo
computed tomography (CT) or magnetic resonance imaging (MRI).
In the setting of cervical spine
trauma, neck immobilization and appropriate imaging studies are
instituted. A patient with lung or breast malignancy, risk factors, or local
or radicular shoulder pain should initially undergo a chest x-ray to
evaluate for chest tumor. A Pancoast tumor is usually caused by
bronchogenic carcinoma and may present, as its first sign, as a Horner's
syndrome.
Neck pain in association with
Horner's syndrome raises the possibility of carotid artery dissection.
Third-order neuronal lesions should also be suspected in those who have
had neck surgery, such as carotid endarterectomy.
Clinical Pearls
1. Patients with Horner's
syndrome demonstrate a narrowed palpebral fissure secondary to a combined
upper eyelid ptosis and higher than normal lower eyelid position
("reverse ptosis"). This is due to loss of sympathetic
innervation to both Müller's muscle of the upper eyelid and as well as to
the inferior tarsal muscle of the lower eyelid.
2. The ptosis of Horner's
syndrome is moderate and never complete.
3. Carotid artery dissection
should be entertained in the patient with neck pain and an ipsilateral
Horner's syndrome.
4. Cluster headaches, with
autonomic sympathetic system dysfunction, are capable of producing an
ipsilateral Horner's syndrome.
|
|
Afferent Pupillary Defect (Marcus Gunn Pupil)
Associated Clinical Features
Pupil size, controlled centrally
by the Edinger-Westphal nucleus in the midbrain, is primarily based on
the afferent light stimulus transmitted via the anterior visual pathway
(Fig. 2.39). These afferents synapse both ipsilaterally and
contralaterally within the midbrain, and pupillomotor fibers within the
oculomotor nerves exit the midbrain to supply the direct and consensual
responses. Even with a unilateral light stimulus, efferents travel to
both pupils and, as a result, pupil size is symmetric. Efferents and
pupil size are thus dependent upon the anterior visual structures,
including the retina, optic nerve, chiasm, optic tract, and midbrain
pathways. A defect anywhere along this pathway modulates the strength of
the afferent light stimulus. An eye with, for example, retinal or optic
nerve disease generates a diminished afferent response because less light
is "perceived"; pupil contraction is decreased as a result.
However, when the contralateral healthy eye is stimulated with light, the
abnormal pupil's consensual response is normal, since the afferent light
stimulus is normal. In this situation the diseased eye is said to
demonstrate an afferent pupillary defect (APD), also called a Marcus Gunn
pupil.
|
|
|
|
|
Afferent
Pupillary Defect Schematic
representation of an afferent pupillary defect (APD) due to
neurologic lesion in the anterior visual pathway.
|
|
A Marcus Gunn pupil is best appreciated by the
swinging flashlight test, which discloses differences in afferent stimuli
between the two eyes. To perform this, a flashlight is directed onto one
pupil and then the other. The normal pupillary response to the flashlight
is a prompt constriction. (Generally this is followed by a slight
"release" dilation.) Normally the brief interval required to
move the light from one eye to the other permits the pupils to begin to
dilate. Thus, under normal circumstances, when the light reaches the
second eye, constriction can again be observed. In the abnormal situation
with a diseased second eye, however, the pupils dilate instead of
constrict (Fig. 2.40). This is because this second eye perceives less
light than the first, and the midbrain therefore sets pupil size to be
larger.
|
|
|
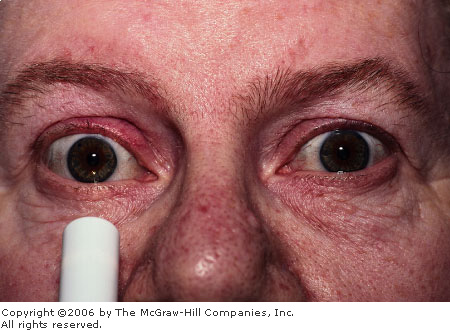
|
|
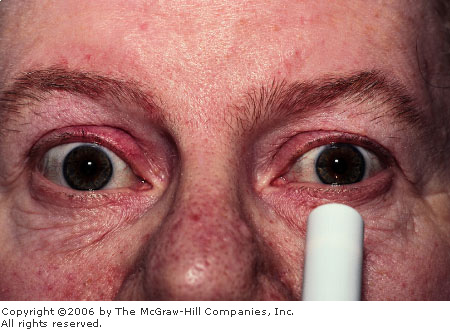
|
|
Marcus
Gunn Pupil These photographs
demonstrate an afferent pupillary defect of the left pupil. When the
light shines in the affected left eye, the pupils are less
constricted than when the light shines in the right eye. Thus, when
the light is swung from the right pupil to the left, the pupils
appear to dilate. The anisocoria here is subtle. (Courtesy of Frank
Birinyi, MD.)
|
|
The swinging flashlight test is
an extremely useful screening technique and one of the most important
assessments for evaluation of optic nerve dysfunction. Although
sensitive, the swinging flashlight test is not specific, since the
pathology may be anywhere in the visual pathway from the retina to the
midbrain. Within the globe, limited pathology such as a cataract or
occlusion of a branch retinal vein or branch retinal artery is unlikely
to produce a clinically detectable APD. However a detectable APD may be
seen in cases of occlusion of the central retinal vein or artery, retinal
detachment, or a dense hemorrhage in the anterior chamber or vitreous. An
APD in the absence of gross ocular disease indicates pathology elsewhere
in the anterior visual pathway, such as a compressive lesion involving
the optic chiasm or tract. Typically, however, a positive finding is
indicative of optic nerve disease. A Marcus Gunn pupil is best seen in
conditions involving the optic nerve, such as ischemic optic neuropathy,
optic neuritis (including that seen with multiple sclerosis), retrobulbar
optic neuritis, and glaucoma.
In patients with an APD,
associated findings may include decreased visual acuity and decreased
visual fields. Funduscopic findings may demonstrate the basis for the
APD; they include occlusion of a central retinal vein or artery, optic
nerve pallor, optic nerve cupping, or severe vitreal hemorrhage.
Differential Diagnosis
An Adie's pupil, more common in
young women, shows a diminished or absent reaction to light. It occurs
with both direct and consensual response. Acutely, the Adie's pupil is
dilated; chronically, it may be constricted. An Argyll-Robertson pupil,
seen in neurosyphilis, is usually bilateral and miotic, it does not
respond to light stimulation, but it does accommodate.
Emergency Department Treatment
and Disposition
The finding of a Marcus Gunn
pupil is nonspecific. A funduscopic examination may provide the basis for
the APD, and ophthalmic consultation is indicated. If the funduscopic
examination is normal, a neurologic evaluation (history, physical
examination, and CT scan) is important to assess for treatable
conditions. In the absence of gross ocular or neurologic disease, a
clinically stable patient may be discharged from the ED with
ophthalmology follow-up.
Clinical Pearls
1. Afferent pupillary defects
do not cause anisocoria because any change in light input results in a
bilaterally symmetric output from the midbrain.
2. Dim room illumination may be
helpful in performing the swinging flashlight test. The patient should
focus on an object 15 ft away to avoid the pupillary constriction
normally seen with accommodation.
3. The normal pupillary
response to bright light is an initial constriction followed by a small
amount of dilation. In performing the swinging flashlight test, it is
important to assess the initial reaction. In the Marcus Gunn pupil, this
initial reaction is dilation.
4. A "subjective" APD
(as can be seen in mild cases of optic neuritis) is demonstrated when no
objective APD is seen but the patient perceives less light in the
affected eye and has mildly decreased visual acuity.
5. If an eye is damaged or
paralyzed or anisocoria is present, an APD can still be assessed by
observing the response of the normal pupil with light that is
shined alternately in each eye. As the light shines in the abnormal eye,
the normal eye dilates if the abnormal eye "perceives"
less light.
|
|
Third-Nerve Palsy
Associated Clinical Features
The third cranial nerve controls
all extraocular muscles (except the lateral rectus and superior oblique)
as well as the levator palpebrae muscle. It also supplies parasympathetic
input to the pupillary constrictor and ciliary muscles. Thus symptoms of
third-nerve palsy include double vision, droopy lid, an enlarged pupil,
and blurred vision at near range. If the ptosis is complete, diplopia may
not be recognized. The clinical examination demonstrates a dilated and
unreactive pupil, limited extraocular movements, and ptosis (Fig. 2.41).
The affected pupil faces laterally (exotropia) secondary to the unopposed
action of the lateral rectus.
|
|
|

|
|
Third-Nerve
Palsy This composite shows the
classic defects of a third cranial nerve palsy in all fields of gaze.
The pupil is dilated. Conjugate eye movement is present in only one
position, when the affected eye gazes laterally to the affected side
(intact lateral rectus). When gaze is directly ahead, exotropia is
seen secondary to the unopposed lateral rectus muscle of the affected
side. (Courtesy of Frank Birinyi, MD.)
|
|
A third-nerve palsy can result
from a lesion anywhere along its anatomic path, which begins in the
brainstem nucleus, continues within the subarachnoid space, traverses the
cavernous sinus, and terminates within the orbit. Associated signs and
symptoms help to delineate a more precise location. An oculomotor palsy
with contralateral hemiplegia suggests brainstem involvement. Infarction
of the midbrain, involving the third nerve and corticospinal tracts (with
contralateral hemiplegia), comprises Weber's syndrome.
In cases of isolated oculomotor
palsy, the subarachnoid space is the most likely site of pathology. On
leaving the brainstem, the nerve enters the subarachnoid space and
travels alongside the posterior communicating artery. A common and
extremely important cause for a third-nerve palsy with pupillary
involvement is a posterior communicating artery aneurysm at the junction
of the posterior communicating and internal carotid arteries. Because of
the dorsal and medial location of the parasympathetic fibers (controlling
pupillary constriction) within the oculomotor nerve bundle, these fibers
are particularly susceptible to compressive lesions, and pupillary
involvement often precedes ophthalmoparesis. With uncal herniation,
enlarging lesions or edema in the middle fossa or temporal lobe push the
medial edge of the uncus toward the midline and over the edge of the
tentorium, compressing the underlying third nerve. Other causes of
oculomotor dysfunction within the subarachnoid space include compressive
neoplasms, inflammatory lesions, and trauma. Typically the trauma is
severe enough to cause a skull fracture and loss of consciousness. An
oculomotor palsy after minor trauma suggests an underlying mass lesion or
aneurysm.
An oculomotor palsy secondary to
pathology within the cavernous sinus may be inferred by associated
involvement of the other structures within the cavernous sinus itself.
These include the trochlear and abducens nerves, the first division of
the trigeminal nerve, sympathetics to the eye, and the venous drainage of
the eye and orbit. Causes for a third-nerve palsy within the cavernous
sinus include neoplasms (pituitary, meningioma, craniopharyngioma,
nasopharyngeal, metastatic), compression by carotid artery aneurysm,
cavernous sinus thrombosis, and carotid-cavernous fistula. A
carotid-cavernous fistula can present abruptly and dramatically as a
result of reversal of blood flow in the orbital and ocular venous
systems. These patients may complain of a bruit and pain. Clinical
findings include pulsatile exophthalmos, elevated intraocular pressure,
and chemosis. Vascular findings may be diffuse or localized. Conjunctival
vessels are dilated and have a corkscrew appearance. A bruit may be
audible by placing the bell of the stethoscope over the closed eyelid.
Cavernous sinus thrombosis presents similarly to carotid-cavernous
fistula, and the patients commonly have manifestations of sepsis. Within
the anterior aspect of the cavernous sinus, the third nerve divides in
two. The superior division innervates the levator palpebrae superioris
and superior rectus muscles, while the inferior division innervates the
medial rectus, inferior rectus, and inferior oblique muscles as well as
the parasympathetics (preganglionic to the ciliary ganglion). Therefore
third-nerve palsies due to cavernous sinus and orbital lesions are
frequently partial, and the pupillomotor fibers may be spared.
Orbital lesions causing
third-nerve palsies may be due to inflammation, trauma, neoplasms, and
mucoceles. Associated clinical findings include proptosis, visual loss,
and sixth-nerve involvement. As noted, an oculomotor palsy secondary to
orbital pathology may be partial. In cases of trauma, the slit-lamp
examination may demonstrate a tear in the iris sphincter.
An isolated third-nerve palsy is
common with diabetic or hypertensive disease. The cause is felt to be
microvascular ischemia. In this situation, the palsy rarely affects the
pupil ("pupil sparing"). Microvascular third-nerve palsies,
especially in diabetics, may be exquisitely painful.
Differential Diagnosis
Unilateral pupil dilation is also
seen with minor eye trauma, eye drops (either alpha-adrenergic agents or
anticholinergics such as atropine), inadvertent contamination from a
scopolamine patch, and an acute Adie's pupil (a dilated and minimally
reactive or nonreactive pupil, seen unilaterally and most often in young
women). Myasthenia gravis, thyroid disease, temporal arteritis, and
orbital inflammatory pseudotumor have similar features. Ptosis is seen in
Horner's syndrome.
Emergency Department Treatment
and Disposition
The evaluation of a patient with
a third-nerve palsy is dependent upon the associated signs and symptoms
that help to localize the pathology. In patients with involvement of the
brainstem, CT or MRI is indicated. Associated fever, headache, depressed
level of consciousness, neck stiffness, or other cranial nerve
involvement should prompt consideration for CT scanning and subsequent
spinal tap. If cavernous sinus involvement is suspected, MRI with
gadolinium is preferred. For orbital pathology, CT scanning with contrast
and thin coronal and axial views is recommended.
Of particular concern is the
sudden onset of a third-nerve palsy accompanied by
"thunderclap" headache, stiff neck, and a depressed level of
consciousness. Even those with pupil sparing should be treated as
neurosurgical emergencies and require immediate evaluation for an
aneurysm or uncal herniation. The workup includes emergent CT or MRI. If
subarachnoid hemorrhage is not found and suspicion of aneurysmal leakage
remains high, a lumbar puncture should be performed. Options include CT
with contrast and magnetic resonance angiography. However, the "gold
standard" remains angiography, since small aneurysms can be missed,
and even small aneurysms can rupture.
In the setting of head trauma, a
patient with third-nerve palsy should undergo measures to reduce the
intracranial pressure. These include elevation of the head of the bed,
mannitol, and burr holes.
Patients under 50 years of age
with isolated third-nerve palsy of any extent should undergo a full
neurologic evaluation, including cerebral angiography. For patients older
than 50 with vasculopathic risk factors who present with isolated
pupil-sparing third-nerve palsies felt to be secondary to an ischemic
neuropathy, the minimum ED evaluation should include measurement of the
blood pressure and glucose. Such patients may be discharged from the ED
provided that they can be observed closely for a week for evidence of
pupillary involvement. Microvascular third-nerve palsies can be
exquisitely painful and thus may require analgesics for 1 to 2 weeks. The
majority of microvascular third-nerve palsies resolve within 3 months.
Clinical Pearls
1. A third-nerve palsy can
result from pathology anywhere along its anatomic pathway, beginning with
the brainstem, continuing within the subarachnoid space, traversing the
cavernous sinus, and terminating within the orbit itself.
2. Patients with the abrupt
onset of a "thunderclap" headache and third-nerve palsy require
immediate neurosurgical evaluation for an aneurysm. (Posterior
communicating artery is a common cause.)
3. In patients over 50 years of
age with pupil-sparing third-nerve palsies, the etiology is usually
hypertensive or diabetic vascular disease.
4. In the patient who presents
with diplopia and possible third-nerve palsy, the ED physician should
confirm the binocularity of the diplopia by monocular occlusion.
Monocular diplopia is optical in origin.
5. A carotid-cavernous fistula
most often (80%) results from trauma. It may be manifest at the time of
injury or delayed for days or weeks. The trauma may be trivial in some
patients.
|
|
Sixth-Nerve Palsy
Associated Clinical Features
The abducens nerve innervates the
lateral rectus muscle and is the most common single-muscle palsy.
Paralysis produces loss of abduction (Fig. 2.42) and horizontal diplopia.
The diplopia is accentuated with gaze toward the affected side. Patients
tend to turn their faces toward the affected eye to limit their diplopia.
Other eye movements are normal; the lid and pupil are not affected.
|
|
|
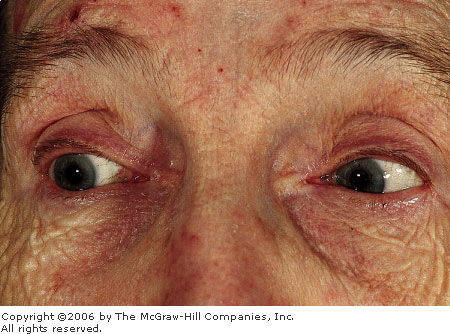
|
|
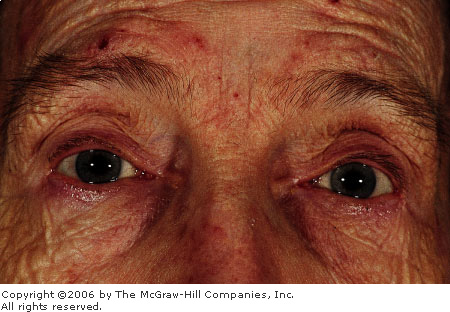
|
|
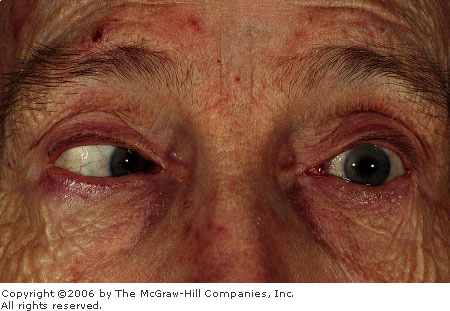
|
|
Sixth-Nerve
Palsy Loss of abduction of the
left eye is seen in lateral gaze, demonstrating an isolated
sixth-nerve palsy. (Courtesy of Frank Birinyi, MD.)
|
|
Other associated findings are
dependent upon the location of the lesion causing the sixth-nerve palsy.
Within the pons, involvement of adjacent structures such as the
corticospinal tract results in contralateral hemiparesis. Wernicke's
encephalopathy affects the abducens nucleus and manifests as ipsilateral
conjugate gaze palsy—i.e., paresis of the ipsilateral lateral
rectus and contralateral medial rectus. This occurs because the abducens
nucleus comprises two populations of neurons. One group forms the sixth
nerve. The other group sends fibers to join the contralateral medial
longitudinal fasciculus, with projection to the medial rectus subnucleus.
As the nerve emerges from the
pons, the abducens ascends the clivus and is vulnerable to compression by
downward or forward movement of the brainstem. In fact, the abducens
nerve has the longest intracranial course of any nerve; it is therefore
vulnerable to "stretching," resulting in sixth-nerve palsies,
unilateral or bilateral, due to elevated intracranial pressure, trauma,
neurosurgical manipulation, and cervical traction. Aneurysmal compression
is much less common than is seen with third-nerve palsy. Any meningeal
process (infectious, inflammatory, or neoplastic) is capable of affecting
this portion of the sixth nerve.
Prior to entering the cavernous
sinus, the abducens nerve crosses the petrous portion of the temporal
bone. Trauma with temporal bone fracture can result in a combination of
sixth- and seventh-nerve palsies, including bilateral palsies. Additional
clinical findings of a basilar skull fracture include hemotympanum,
Battle's sign, and leakage of CSF or blood from the external ear canal.
Pathology within the cavernous
sinus can affect, in addition to the abducens nerve, the internal carotid
artery, the venous drainage of the eye and orbit, trochlear and
oculomotor nerves, the first division of the trigeminal nerve, and the
ocular sympathetics. The abducens tends to be particularly involved with
vascular lesions within the cavernous sinus because of its adjacent
position inferolateral to the carotid. A carotid-cavernous fistula can
present abruptly and dramatically as a result of reversal of blood flow
in the orbital and ocular venous systems. These patients may complain of
a bruit and pain. Clinical findings include pulsatile exophthalmos,
elevated intraocular pressure, and chemosis. Vascular findings may be
diffuse or localized. Conjunctival vessels are dilated and have a
corkscrew appearance. A bruit may be audible when the bell of the
stethoscope is placed over the closed eyelid.
Within the orbit itself, isolated
involvement of the sixth nerve is rare because of its short course prior
to innervating the lateral rectus muscle.
The abducens nerve may be
infarcted by microvascular changes secondary to diabetes, hypertension,
giant cell arteritis, or arteriosclerosis. In children, a transient
sixth-nerve palsy may follow a virus infection.
Differential Diagnosis
Thyroid eye disease, orbital
inflammatory pseudotumor, myasthenia gravis, Duane's syndrome (congenital
absence of the sixth-nerve nucleus), Parinaud's syndrome (dorsal midbrain
syndrome), and medial rectus entrapment secondary to an ethmoid fracture
have similar findings.
Emergency Department Treatment
and Disposition
The ED evaluation is guided by
the associated signs and symptoms. With involvement of the brainstem or
cavernous sinus, CT or MRI is indicated. Pathology localized to the
subarachnoid space should prompt consideration for CT scanning and
subsequent spinal tap.
Children with antecedent viral
illness and normal CT scan may be discharged provided that close
follow-up is arranged. Children usually recover completely within 4
months. In the elderly, an isolated sixth-nerve palsy is likely ischemic,
transient, and not indicative of underlying neurologic disease. In these
cases, a glucose and an erythrocyte sedimentation rate (for evidence of
giant cell arteritis) are appropriate. Although the palsy generally
resolves in 3 to 4 months, these patients should be followed up carefully
for the onset of additional neurologic abnormalities.
There is no treatment for the
palsy itself except for patching the affected eye if diplopia is
bothersome.
Clinical Pearls
1. Isolated sixth-nerve palsy
is commonly due to microvascular disease, not an aneurysm.
2. An isolated lateral rectus
palsy should not be considered nuclear in origin.
3. A deficit involving a
sixth-nerve palsy with an ipsilateral Horner's syndrome is usually
localized to the cavernous sinus, since sympathetic fibers, as they
traverse from the internal carotid artery to the oculomotor nerve, may
briefly accompany the abducens nerve.
4. Basilar skull fractures of
the temporal bone are capable of producing a traumatic sixth-nerve palsy,
since the nerve passes from the posterior fossa to the cavernous sinus
via the petrous ridge.
|
|