|
Note: Large images and
tables on this page may necessitate printing in landscape mode.
Copyright
©2006 The McGraw-Hill Companies. All rights reserved.
Emergency
Medicine Atlas > Part 1. Regional
Anatomy > Chapter 3. Funduscopic Findings >
|
Normal Fundus (Disk, Macular Reflex, Background)
Associated Clinical Features
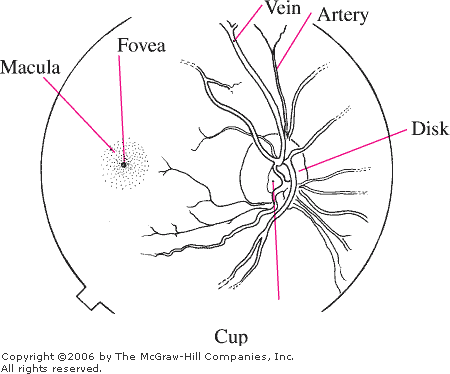
Disk
The disk is pale pink,
approximately 1.5 mm in diameter, with sharp, flat margins (Fig. 3.1).
The physiologic cup is located within the disk and usually measures less
than six-tenths the disk diameter. The cups should be approximately equal
in both eyes.
|
|
|
|
|
|
|
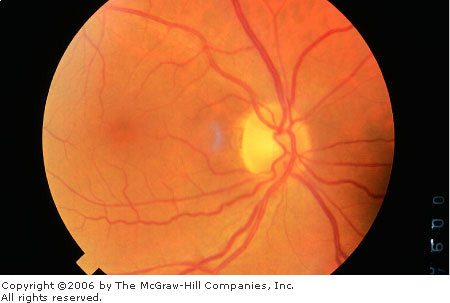
Normal
Fundus The disk has sharp
margins and is normal in color, with a small central cup. Arterioles
and venules have normal color, sheen, and course. Background is in
normal color. The macula is enclosed by arching temporal vessels. The
fovea is located by a central pit. (Courtesy of Beverly C. Forcier, MD.)
|
|
Vessels
The central retinal artery and
central retinal vein travel within the optic nerve, branching near the
surface into the inferior and superior branches of arterioles and
venules, respectively. Normally the walls of the vessels are not visible;
the column of blood within the walls is visualized. The venules are seen
as branching, dark red lines. The arterioles are seen as bright red
branching lines, approximately two-thirds or three-fourths the diameter
of the venules.
Macula
This is an area of the retina
located temporal to the disk; it is void of capillaries. The fovea is an
area of depression approximately 1.5 mm in diameter (similar to the optic
disk) in the center of the macula. The foveola is a tiny pit located in
the center of the fovea. These areas correspond to central vision.
Background
The background fundus is red;
there is some variation in the color, depending on the amount of
individual pigmentation and the visibility of the choroidal vessels
beneath the retina.
Clinical Pearls
1. Fundal examination should be
an integral part of any eye examination.
2. The cup/disk ratio is
slightly larger in the African-American population.
3. The normal fundus should be
void of any hemorrhages, exudates, or tortuous vasculature.
|
|
Age-Related Macular Degeneration
Associated Clinical Features
Age-related macular degeneration,
the leading cause of blindness in the elderly, increases in incidence
with each decade over 50. Degeneration of the macula may be evidenced by
accumulation of either drusen (small, discrete, round, punctate nodules),
or soft drusen (larger, pale yellow or gray, without discrete margins
that may be confluent) (Fig. 3.2A and B). Most patients with drusen have
good vision, although there may be decreased visual acuity and distortion
of vision. There may be associated pigmentary changes and atrophy of the
retina. Vision may slowly deteriorate if atrophy occurs.
Patients with early or late
degenerative changes of the macula are at risk of developing subretinal
neovascularization (SRNV), which is associated with distortion of vision,
blind spots, and decreased visual acuity. Macular appearance may show
dirty gray lesions, hemorrhage, retinal elevation, and exudation (Fig.
3.3).
|
|
|

|
|
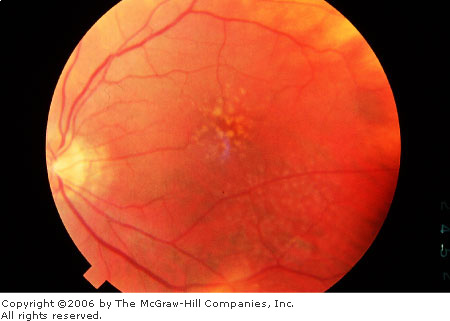
Age-Related
Macular Degeneration, Drusen
Typcial macular drusen and retinal pigment epithelial (RPE) atrophy
(scalloped pigment loss) in age-related macular degeneration.
(Courtesy of Richard E. Wyszynski, MD.)
|
|
|
|
|
|
|
Age-Related
Macular Degeneration, Drusen
Drusen are clustered in the center of the macula. (Courtesy of
Richard E. Wyszynski, MD.)
|
|
|
|
|
|
|
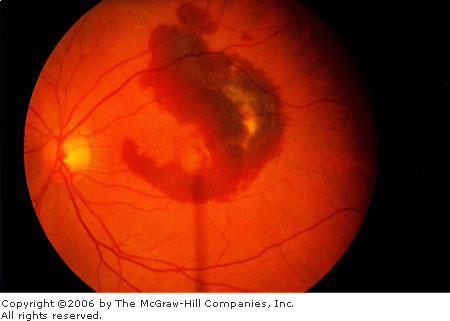
Age-Related
Macular Degeneration Hemorrhage
seen beneath the retina in association with subretinal
neovascularization. (Courtesy of Richard E. Wyszynski, MD.)
|
|
Differential Diagnosis
Hereditary macular degenerations,
other acquired macular disorders including toxicities, and retinal
exudation may present similarly.
Hemorrhages and exudates can
present from vascular disease, ocular disorders such as inflammations or
infections, tumors, trauma, and hereditary disorders.
Emergency Department Treatment
and Disposition
Patients with drusen need ophthalmologic
evaluation every 6 to 12 months or sooner if visual distortion or
decreasing visual acuity develops. If a patient complains of
deterioration of visual acuity or image distortion, prompt ophthalmic
evaluation is warranted, probably including fluorescein angiography. If
SRNV is present, laser treatment may be indicated.
Clinical Pearls
1. Age-related macular
degeneration is the leading cause of blindness in the United States in
patients above 65 years of age.
2. Patient may have normal
peripheral vision.
3. Untreated SRNV can lead to
visual loss within a few days.
4. Patients frequently complain
of distortion with SRNV.
|
|
Exudate
Associated Clinical Features
Hard exudates (Figure 3.4A) are
refractile, yellowish deposits with sharp margins composed of fat-laden
macrophages and serum lipids. Occasionally the lipid deposits form a
partial or complete ring (called a circinate ring) around the leaking
area of pathology. If the lipid leakage is located near the fovea, a
spoke, or star-type distribution of the hard exudates is seen.
Cotton wool spots, or soft
"exudates," are actually microinfarctions of the retinal
nerve-fiber layer, and appear white with soft or fuzzy edges (Fig. 3.4B).
Inflammatory exudates are
secondary to retinal or chorioretinal inflammation.
|
|
|
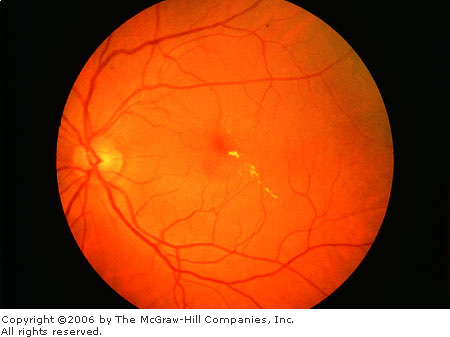
|
|
Hard
Exudates Linear collection of
yellow lipid deposits with sharp margins in macula. (Courtesy of
Beverly C. Forcier, MD.)
|
|
|
|
|
|
|
Cotton
Wool Spots White lesions with
fuzzy margins, seen here approximately one-fifth to one-fourth disk
diameter in size. Orientation of cotton wool spots generally follows
the curvilinear arrangement of the nerve fiber layer. Intraretinal
hemorrhages and intraretinal vascular abnormalities are also present.
(Courtesy of Richard E. Wyszynski, MD.)
|
|
Differential Diagnosis
Hard exudation and cotton wool
spots are associated with vascular diseases such as diabetes mellitus,
hypertension, and collagen vascular diseases but can be seen with
papilledema and other ocular conditions. Inflammatory exudates are seen
in patients with such diseases as sarcoidosis and toxoplasmosis.
Emergency Department Treatment
and Disposition
Routine referral for
ophthalmologic and medical workup is appropriate.
Clinical Pearl
1. Hard exudates that are
intraretinal may easily be confused with drusen occurring near Bruch's
membrane, which separates the retina from the choroid.
|
|
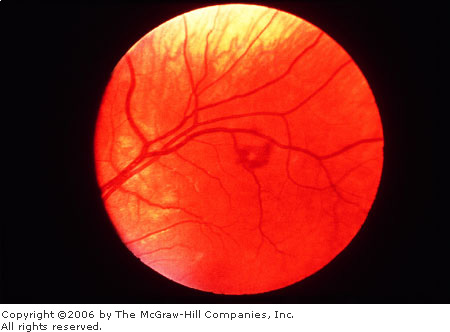
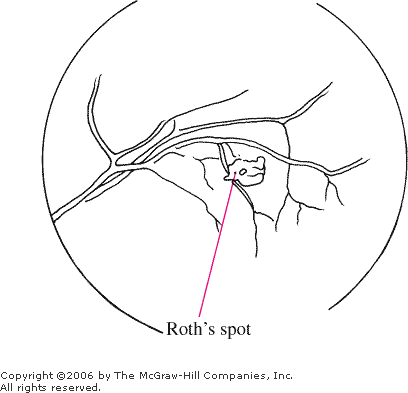
Roth's Spot
Associated Clinical Features
Roth's spots are retinal
hemorrhages with a white or yellow center (Fig. 3.5). They are seen in
patients with a host of diseases such as anemia, leukemia, multiple
myeloma, diabetes mellitus, collagen vascular disease, other vascular
disease, intracranial hemorrhage in infants, septic retinitis, and lung
carcinoma.
|
|
|
|
|
|
|
Roth's
Spot Retinal hemorrhage with
pale center. (Courtesy of William E. Cappaert, MD.)
|
|
Differential Diagnosis
Flamed-shaped or splinter
hemorrhages or dot-blot hemorrhages may resemble Roth's spots.
Emergency Department Treatment
and Disposition
Routine referral for general
medical evaluation is appropriate.
Clinical Pearls
1. Roth's spots are not
pathognomonic for any particular disease process and can represent a
variety of clinical conditions.
2. These lesions represent red
blood cells surrounding inflammatory cells.
|
|
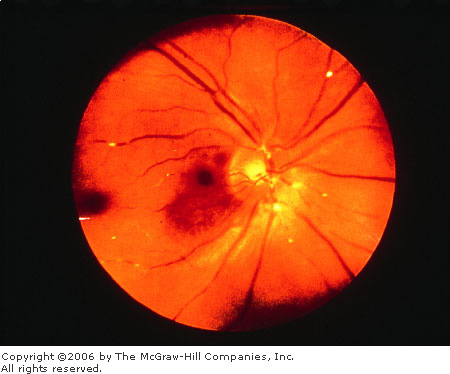
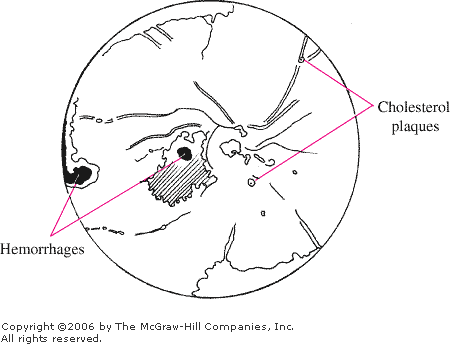
Emboli
Associated Clinical Features
Plaques, if present, are often
found at arteriolar bifurcations (Fig. 3.6). Patients may have signs and
symptoms of vascular disease including a "source" of emboli
such as carotid bruits or stenosis, aortic stenosis, aneurysms, or atrial
fibrillation. Amaurosis fugax, a transient loss of vision often described
as a curtain of darkness obscuring vision with sight restoration within a
few minutes, may be present in the history.
|
|
|
|
|
|
|
Emboli Refractile cholesterol plaques usually lodge
at vessel bifurcations. (Courtesy of William E. Cappaert, MD.)
|
|
Differential Diagnosis
Cholesterol emboli (Hollenhorst
plaques), associated with generalized atherosclerosis often from carotid
atheroma, are bright, highly refractile plaques; platelet emboli (carotid
artery or cardiac thrombus) are white and very difficult to visualize;
and calcific emboli (cardiac valvular disease) are irregular and white or
dull gray and much less refractile.
Emergency Department Treatment
and Disposition
Referral for routine general
medical evaluation is appropriate unless the patient presents with signs
or symptoms consistent with showering of emboli, transient ischemic
attack, or cerebrovascular accident, in which case referral for admission
is indicated.
Clinical Pearls
1. Retinal emboli may produce a
loss of vision, either transient or permanent in nature.
2. Arteriolar occlusion may
occur either in a central or peripheral branch location.
|
|
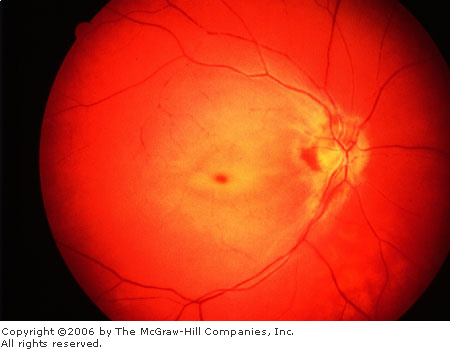
Central Retinal Artery Occlusion (CRAO)
Associated Clinical Features
The typical patient experiences a
sudden, painless monocular loss of vision, either segmental or complete.
Visual acuity may range from finger counting or light perception to
complete blindness. Fundal findings include: fundal paleness due to
retinal edema; the fovea does not have the edema and thus appears as a
cherry-red spot; the retinal arterioles are narrow and irregular; and the
retinal venules have a "boxcar" appearance (Fig. 3.7).
|
|
|
|
|
Central
Retinal Artery Occlusion The
retinal pallor due to retinal edema is well demonstrated, contrasting
with the "cherry red spot" of the nonedematous fovea. Note
the vascular narrowing and the "boxcar" appearance of the
venules. (Courtesy of Richard E. Wyszynski, MD.)
|
|
Differential Diagnosis
Arteriosclerosis, arterial
hypertension, carotid artery disease, diabetes mellitus, and valvular
heart disease are the most common systemic disorders associated with
central retinal artery occlusion (CRAO). Other associated disorders
include vascular disorders, trauma, and coagulopathies. Temporal
arteritis may present with similar visual complaints.
Emergency Department Treatment
and Disposition
Attempts to restore retinal blood
flow may be beneficial if performed in a very narrow time window after
the acute event. This may be accomplished by (1) decreasing intraocular
pressure with topical beta-blocker eye drops or intravenous
acetazolamide; (2) ocular massage, applied with cyclic pressure on the
globe for 10 s, followed by release and then repeated. Urgent
consultation with an ophthalmologist if the CRAO is less than a few hours
old is indicated to determine if more aggressive acute therapy
(paracentesis) is warranted. However, such aggressive treatment rarely
alters the poor prognosis. Medical evaluation and treatment of associated
findings may be warranted.
Clinical Pearls
1. History should focus on how
long ago the episode occurred. If the loss of vision occurred recently,
then the patient should be triaged and examined quickly so as to consult
an ophthalmologist within the treatment window.
2. Sudden, painless monocular
vision loss is typical.
3. CRAO may be associated with
temporal arteritis. This diagnosis should be strongly considered in all
patients presenting with signs and symptoms of CRAO who are older than 55
years.
|
|
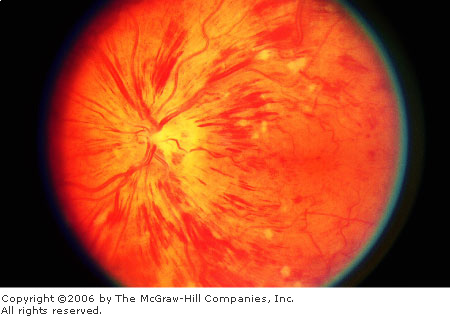
Central Retinal Vein Occlusion (CRVO)
Associated Clinical Features
Patients are usually older
individuals and complain of sudden, painless visual loss in one eye. The
vision loss is usually not as severe as CRAO and may vary from normal to
hand motion. Funduscopy in a classic, ischemic central retinal vein
occlusion (CRVO) shows a "blood and thunder" fundus:
hemorrhages (including flame, dot or blot, preretinal, and vitreous) and
dilation and tortuosity of the venous system. The arterial system often
shows narrowing. The disk margin may be blurred. Cotton wool spots and
edema may be seen (Fig. 3.8).
|
|
|
|
|
Central
Retinal Vein Occlusion The
amount of hemorrhage is the most striking feature in this photograph.
Also note the blurred disk margin, the dilation and tortuosity of the
venules, and the cotton wool spots. Retinal edema is suggested by
blurring of the retinal details. (Courtesy of Department of
Ophthalmology, Naval Medical Center, Portsmouth, VA.)
|
|
Differential Diagnosis
Retinal detachment, papilledema,
and central retinal artery occlusion can have a similar appearance.
Emergency Department Treatment
and Disposition
Treatment is rarely effective in
preventing or reversing the damage done by the occlusion and is directed
toward systemic evaluation to identify and treat contributing factors,
hopefully decreasing the chance of contralateral CRVO. Ophthalmologic
evaluation is necessary to confirm the diagnosis, estimate the amount of
ischemia, and follow the patient so as to minimize sequelae of possible
complications such as neovascularization and neovascular glaucoma.
Clinical Pearls
1. Sudden, painless visual loss
in one eye should be evaluated promptly to determine its etiology.
2. Look for the classic
"blood and thunder" funduscopic findings.
3. Consider the differential
diagnosis of acute painful (glaucoma, retrobulbar neuritis) versus
painless vision loss (CRAO, anterior ischemic optic neuropathy,
retinal detachment, subretinal neovascularization, and vitreous
hemorrhage).
|
|
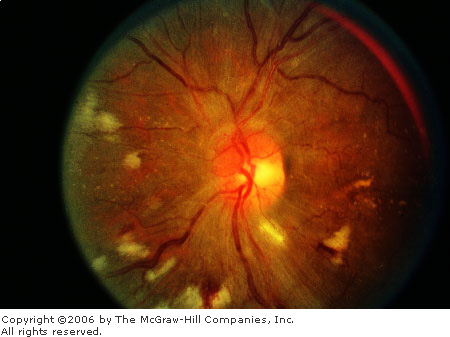
Hypertensive Retinopathy
Associated Clinical Features
Fundus changes that may be seen
with hypertension include generalized and focal narrowing of arterioles,
generalized arteriolar sclerosis (resembling copper or silver wiring),
arteriovenous crossing changes, hemorrhages (usually flame-shaped),
retinal edema and exudation, cotton wool spots, microaneurysms, and disk
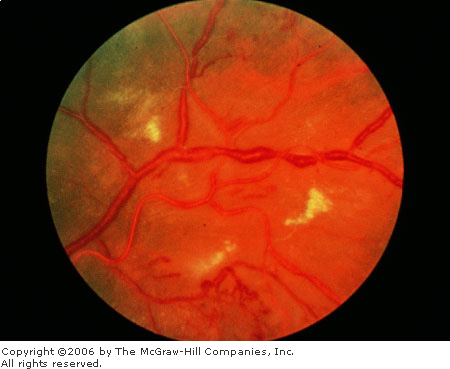
edema (Fig. 3.9).
|
|
|
|
|
Hypertension Chronic, severe systemic hypertensive changes
are demonstrated by hard exudates, increased vessel light reflexes,
and sausage-shaped veins. (Courtesy of Richard E. Wyszynski, MD.)
|
|
Differential Diagnosis
Diabetic retinopathy, many
hemopoietic and vascular diseases, traumas, localized ocular pathology,
and papilledema should all be considered.
Emergency Department Treatment
and Disposition
Medical treatment of
hypertension.
Clinical Pearls
1. Hypertensive arteriolar
findings may be reversible if organic changes have not occurred in the
vessel walls.
2. Always consider hypertensive
retinopathy in the differential diagnosis of papilledema.
|
|
Diabetic Retinopathy
Associated Clinical Features
The early ocular manifestations
of diabetes mellitus are referred to as background diabetic retinopathy
(BDR). Fundus findings include flame or splinter hemorrhages (located in
the superficial nerve fiber layer) or dot and blot hemorrhages (located
deeper in the retina), hard exudates, retinal edema, and microaneurysms
(Fig. 3.10A and B). If these signs are located in the macula, the
patient's visual acuity may be decreased or at risk of becoming
compromised, requiring laser treatment. Preproliferative diabetic
retinopathy can show BDR changes plus cotton wool spots, intraretinal
microvascular abnormalities, and venous beading. Proliferative diabetic
retinopathy is demonstrated by neovascularization at the disk (NVD) or
elsewhere (NVE) (Fig. 3.10C). These require laser therapy owing to risk
of severe visual loss from sequelae: vitreous hemorrhage, tractional
retinal detachment, severe glaucoma.
|
|
|
|
|
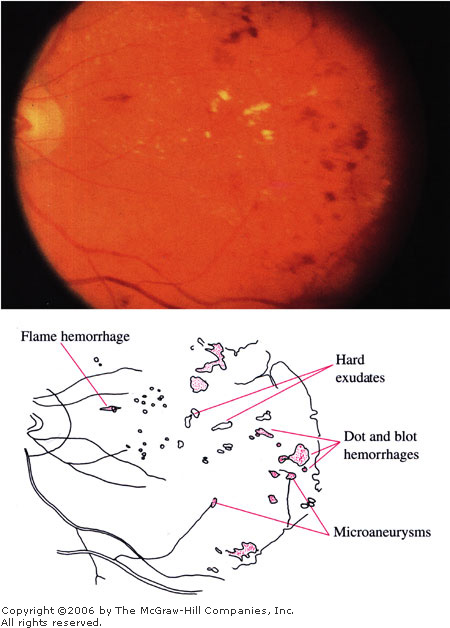
Background
Diabetic Retinopathy Hard
exudates, dot hemorrhages, blot hemorrhages, flame hemorrhages, and
microaneurysms are present. Because these changes are located within
the macula, this is classified as diabetic maculopathy. (Courtesy of
Richard E. Wyszynski, MD.)
|
|
|
|
|
|
|
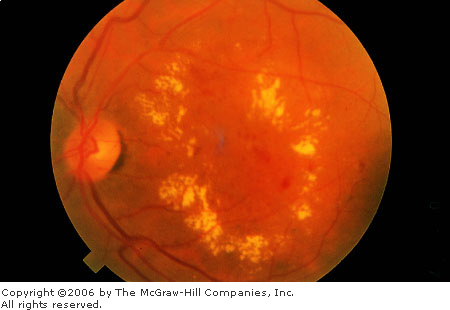
Background
Diabetic Retinopathy An
example of diabetic maculopathy with a typical circinate lipid ring.
(Courtesy of Richard E. Wyszynski, MD.)
|
|
|
|
|
|
|
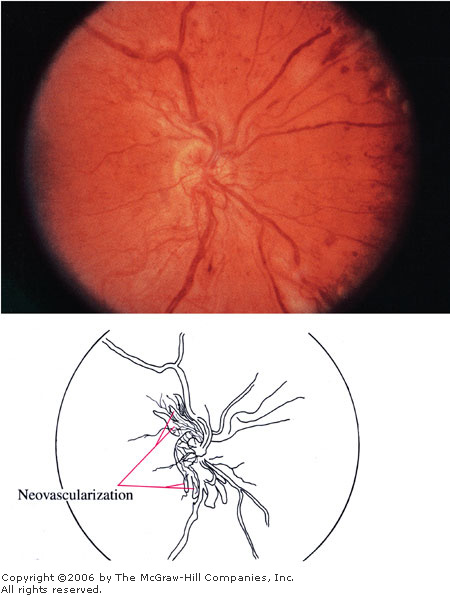
Proliferative
Diabetic Retinopathy In
addition to the signs seen in background and preproliferative
diabetic retinopathy, neovascularization is seen here coming off the
disk. (Courtesy of Richard E. Wyszynski, MD.)
|
|
Differential Diagnosis
Many vascular and hemopoietic
diseases—such as collagen vascular disease, sickle cell trait,
hypertension, hypotension, anemia, leukemia, inflammatory and infectious
states—and ocular conditions can be associated with some of or all
the above signs.
Emergency Department Treatment
and Disposition
Routine ophthalmologic referral
for laser or surgical treatment is indicated.
Clinical Pearls
1. Periodic ophthalmologic
evaluations are recommended.
2. Microaneurysms typically
appear 10 years after the initial onset of diabetes, although they may appear
earlier in patients with juvenile diabetes.
3. Control of blood sugar alone
does not prevent the development of vasculopathy.
4. Blurred vision can also
occur from acute increases in serum glucose, causing lens swelling and a
refractive shift even in the absence of retinopathy.
|
|
Vitreous Hemorrhage
Associated Clinical Features
Patients may complain of sudden
loss or deterioration of vision in the affected eye, although bilateral
hemorrhage can occur. The red reflex is diminished or absent, and the retina
is obscured because of the bleeding. Large sheets or three-dimensional
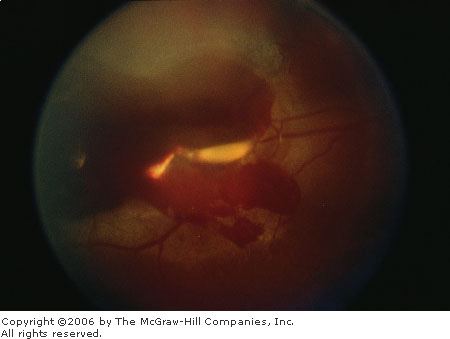
collections of red to red-black blood may be detected (Fig. 3.11A and B).
|
|
|
|
|
Vitreous
Hemorrhage Large amount of
vitreous hemorrhages associated with metallic intraocular foreign
body. The large quantity of blood obscures visualization of retinal
details. (Courtesy of Richard E. Wyszynski, MD.)
|
|
|
|
|

|
|
Vitreous
Hemorrhage A smaller amount of
vitreous hemorrhage is more easily photographed. Gravitational effect
on the vitreous blood creates the appearance of a flat meniscus
(keel-shaped blood) in this patient with vitreous hemorrhage
associated with proliferative diabetic retinopathy. (Courtesy of
Richard E. Wyszynski, MD.)
|
|
Differential Diagnosis
Multiple underlying etiologies
include proliferative diabetic retinopathy, retinal or vitreous
detachments, hematologic diseases, trauma (ocular or shaken impact
syndrome), subarachnoid hemorrhage, collagen vascular disease,
infections, macular degeneration, and tumors.
Emergency Department Treatment
and Disposition
Refer to an ophthalmologist and
an appropriate physician for associated conditions. Ophthalmic
observation, photocoagulation, and surgery are all therapeutic options.
Bed rest may help to increase visualization of the fundus.
Clinical Pearl
1. The patient's vision may
improve somewhat after a period of sitting or standing as the blood
layers out.
|
|
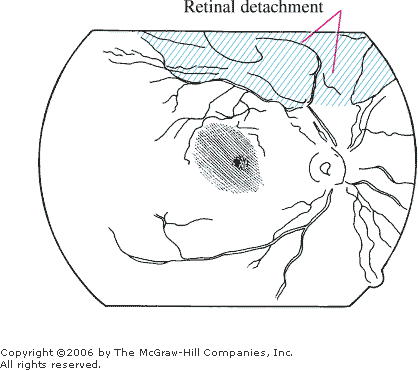
Retinal Detachment
Associated Clinical Features
Patients often complain of
monocular decreased visual function and may describe a shadow or curtain
descending over the eye. Other complaints include cloudy or smoky vision,
floaters, or flashes of light. Central visual acuity is diminished with
macular involvement. Fundal examination may reveal a billowing or
tentlike elevation of retina compared with adjacent areas. The elevated
retina often appears gray. Retinal holes and tears may be seen, but often
the holes, tears, and retinal detachment cannot be seen without indirect
ophthalmoscopy (Fig. 3.12).
|
|
|
|
|
|
|
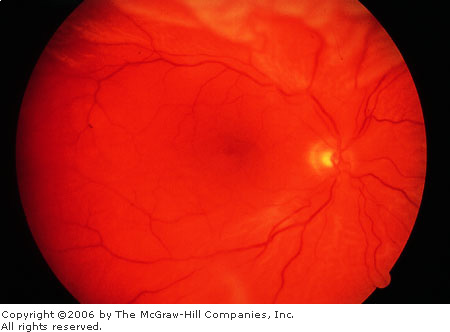
Retinal
Detachment A red, flat,
well-focused macula is contrasted with the pale, undulating,
out-of-focus, elevated retina surrounding the macula. (Courtesy of
Richard E. Wyszynski, MD.)
|
|
Differential Diagnosis
Retinal detachments due to retinal
tears or holes can be associated with trauma, previous ocular surgery,
nearsightedness, family history of retinal detachment, and Marfan's
disease. Retinal detachments due to traction on the retina by an
intraocular process can be due to systemic influences in the eye, such as
diabetes mellitus or sickle cell trait. Occasionally retinal detachments
are due to tumors or exudative processes that elevate the retina.
Symptoms of "light flashes" may occur with vitreous changes in
the absence of retinal pathology. Patients may note flashes of light
occurring only in a darkened environment because of the mechanical
stimulation of the retina from the extraocular muscles, usually in a
nearsighted individual.
Emergency Department Treatment
and Disposition
Urgent ophthalmologic evaluation
and treatment are warranted.
Clinical Pearls
1. Often patients have had
sensation of flashes of light that occur in a certain area of a visual
field in one eye, corresponding to the pathologic pulling on the
corresponding retina.
2. Visual loss may be gradual
or sudden.
|
|
Cytomegalovirus (CMV) Retinitis
Associated Clinical Features
Patients may complain of the
gradual onset of the following visual sensations: floaters, scintillating
scotomas (quivering blind spots), decreased peripheral visual field, and
metamorphopsia (wavy distortion of vision). Cytomegalovirus (CMV)
infiltrates appear as focal, small white lesions in the retina that look
like cotton wool spots. CMV is a necrotizing virus that is spread
hematogenously, so that damage is concentrated in the retina adjacent to
the major vessels and the optic disk. Often hemorrhage is involved with
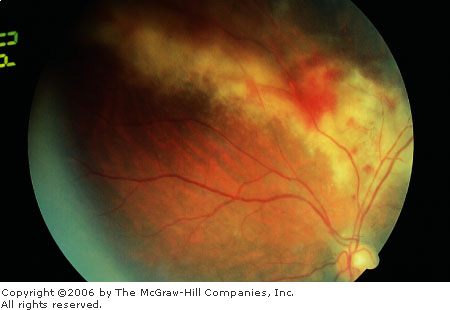
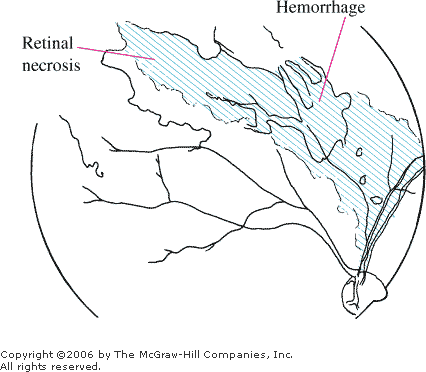
significant retinal necrosis (dirty white with a granular appearance),
giving the "pizza pie" or "cheese and ketchup"
appearance (Fig. 3.13). Optic nerve involvement and retinal detachments
can be present.
|
|
|
|
|
|
|
CMV
Retinitis "Pizza
pie" or "cheese and ketchup" appearance is
demonstrated by hemorrhages and the dirty, white, granular-appearing
retinal necrosis adjacent to major vessels. (Courtesy of Richard E.
Wyszynski, MD.)
|
|
Differential Diagnosis
The differential includes other
infections such as toxoplasmosis, other herpesviruses, syphilis, and
occasionally other opportunistic infections.
Emergency Department Treatment
and Disposition
Reversal, if possible, of
immunosuppression. Ganciclovir and foscarnet have been used with some
effectiveness.
Clinical Pearls
1. HIV retinopathy consists of
scattered retinal hemorrhages and scattered, multiple cotton wool spots
that resolve over time, whereas CMV lesions will typically progress.
2. Although exposure to the CMV
virus is widespread, the virus rarely produces a clinically recognized
disease in nonimmunosuppressed individuals.
|
|
Papilledema
Associated Clinical Features
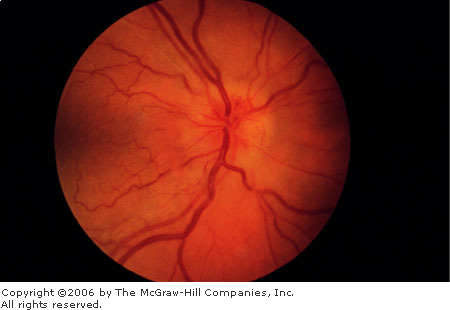
Papilledema involves swelling of
the optic nerve head, usually in association with elevated intracranial
pressure. The optic disks are hyperemic with blurred disk margins; the
venules are dilated and tortuous. The optic cup may be obscured by the
swollen disk. There may be flame hemorrhages and infarctions (white,
indistinct cotton wool spots) in the nerve fiber layer and edema in the
surrounding retina (Fig. 3.14).
|
|
|
|
|
|
|
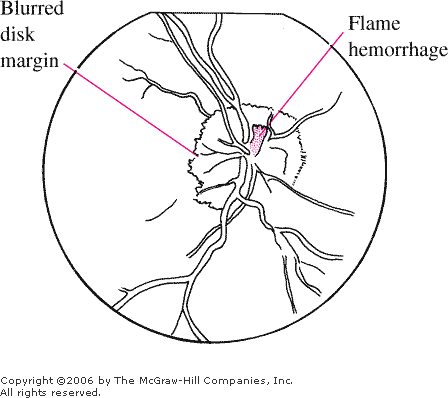
Papilledema Disk is hyperemic and swollen with loss of
sharp margins. The venules are dilated and tortuous. The cup is
obscured. A small flame hemorrhage is seen at 12 to 1 o'clock on the
disk margin. (Courtesy of Department of Ophthalmology, Naval Medical
Center, Portsmouth, VA.)
|
|
Differential Diagnosis
Ocular inflammation (e.g.,
papillitis), tumors or trauma, central retinal artery or vein occlusion,
optic nerve drusen, and marked hyperopia may present with similar
findings.
Emergency Department Treatment
and Disposition
Expeditious ophthalmologic and
medical evaluation is warranted.
Clinical Pearls
1. The top of a swollen disk
and the surrounding unaffected retina will not both be in focus on the
same setting on direct ophthalmoscopy.
2. Papilledema is a bilateral
process, though it may be slightly asymmetric. A unilateral swollen disk
suggests a localized ocular or orbital process.
3. Vision is usually normal
acutely, though the patient may complain of transient visual changes. The
blind spot is usually enlarged.
4. Diplopia from a sixth
cranial nerve palsy can be associated with increased intracranial
pressure and papilledema.
|
|
Optic Neuritis
Associated Clinical Features
Most cases of optic neuritis are
retrobulbar and involve no changes in the fundus, or optic disk, during
the acute episode. With time, variable optic disk pallor may develop
(Fig. 3.15). Typical retrobulbar optic neuritis presents with sudden or
rapidly progressing monocular vision loss in patients younger than 50
years. There is a central visual field defect that may extend to the
blind spot. There is pain on movement of the globe. The pupillary light
response is diminished in the affected eye. Over time the vision improves
partially or completely; minimal or severe optic atrophy may develop.
Papillitis, inflammation of the intraocular portion of the optic nerve,
will accompany disk swelling, with a few flame hemorrhages and possible
cells in the vitreous.
|
|
|
|
|
|
|
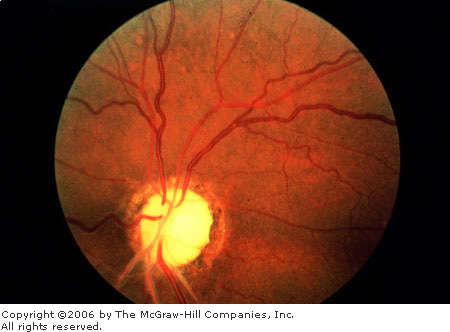
|
|
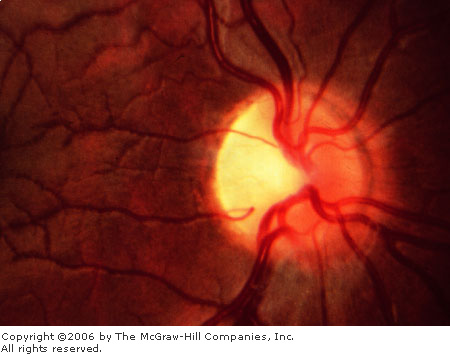
Optic
Nerve Pallor Optic nerve
pallor, either segmental (top) or generalized (bottom),
is a nonspecific change that may be associated with a previous
episode of optic neuritis or other insults to the optic nerve.
(Courtesy of Richard E. Wyszynski, MD.)
|
|
Differential Diagnosis
Optic neuritis must be
differentiated from papilledema (bilateral disk swelling, typically with
no acute visual loss with the exception of transient visual changes),
ischemic neuropathy (pale, swollen disk in an older individual with sudden
monocular vision loss), tumors, metabolic or endocrine disorders. Most
cases of optic neuritis are of unknown etiology. Some known causes of
optic neuritis include demyelinating disease, infections (including
viral, syphilis, tuberculosis, sarcoidosis), or inflammations from
contiguous structures (sinuses, meninges, orbit).
Emergency Department Treatment
and Disposition
Treatment is controversial; often
none is recommended. Oral steroids may worsen prognosis in certain cases.
Intravenous steroids may be considered after consultation with an
ophthalmologist.
Clinical Pearls
1. Monocular vision loss with
pain on palpation of the globe or with eye movement are clinical clues to
the diagnosis.
2. Sudden or rapidly
progressing central vision loss is characteristic.
3. Most cases of acute optic
neuritis are retrobulbar. Thus ophthalmoscopy shows a normal fundus.
|
|
Anterior Ischemic Optic Neuropathy (AION)
Associated Clinical Features
Anterior ischemic optic
neuropathy (AION) presents with a sudden loss of visual field (often
altitudinal), usually involving fixation, in an older individual. The
loss is usually stable after onset, with no improvement, and only
occasionally, progressive over several days to weeks. Pale disk swelling
is present involving a sector or the full disk, with accompanying flame
hemorrhages (Fig. 3.16).
|
|
|
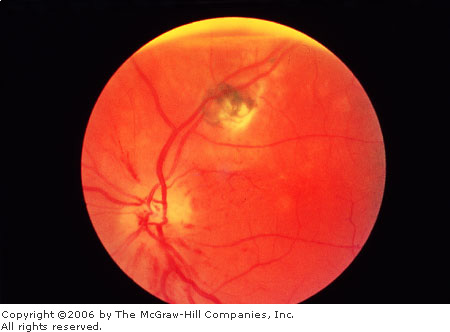
|
|

|
|
Anterior
Ischemic Optic Neuropathy Pale
disk swelling and flame hemorrhages are present. This patient also
has an unrelated retinal scar due to toxoplasmosis. (Courtesy of
William E. Cappaert, MD.)
|
|
Differential Diagnosis
The common, nonarteritic causes
of AION (probably arteriosclerosis) need to be differentiated from
arteritic ones, such as giant cell arteritis. If untreated, the latter
will involve the other eye in 75% of cases, often in a few days to weeks.
These elderly individuals often have weight loss, masseter claudication,
weakness, myalgias, elevated sedimentation rate, and painful scalp,
temples, or forehead.
Emergency Department Treatment
and Disposition
Routine ophthalmologic and
medical evaluation is appropriate.
Clinical Pearls
1. Consider AION in an elderly
patient with sudden, usually painless visual field loss.
2. Rule out giant cell
arteritis. These patients tend to be older (age > 55) and may have associated
CRAO or cranial nerve palsies (III, IV, or VI) with diplopia.
|
|
Glaucoma
Associated Clinical Features
Narrow or closed-angle glaucoma
results from a physical appositional impedance of aqueous humor outflow.
Symptoms range from complaints of colored halos around lights and blurred
vision to severe pain (may be described as a headache or brow ache)
associated with nausea and vomiting. Intraocular pressures are markedly
elevated. Perilimbal vessels are injected, the pupil is middilated and
poorly reactive to light, and the cornea may be hazy and edematous (see
also Chap. 2 for external ocular images).
Two-thirds of glaucoma patients
have open-angle glaucoma due to an abnormality of primary tissues
responsible for the outflow of fluid out of the eye. Often they are
asymptomatic. They may have a family history of glaucoma. Funduscopy may
show asymmetric cupping of the optic nerves (Fig. 3.17). The optic nerve
may show notching, local thinning of tissue, or disk hemorrhage. Optic
cups enlarge, especially vertically, with progressive damage. Tissue loss
is associated with visual field abnormalities, usually in arcuate
patterns. The intraocular pressure is often but not always greater than
21 mmHg.
|
|
|
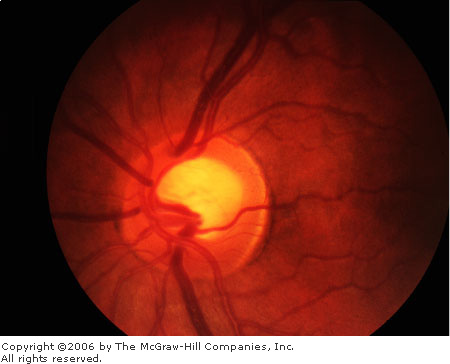
|
|
Glaucomatous
Cupping The cup is not
central; it is elongated toward the rim superotemporally. (Courtesy
of Department of Ophthalmology, Naval Medical Center, Portsmouth, VA.)
|
|
Differential Diagnosis
Acute Glaucoma
Painful visual loss (retrobulbar
optic neuritis, iritis, endophthalmitis), referred pain from
nonophthalmic source.
Chronic Glaucoma
Normal variants, ocular
conditions like disk drusen or optic neuropathy.
Emergency Department Treatment
and Disposition
Acute Narrow-Angle Glaucoma
Emergent ophthalmologic
consultation and administration of medications to decrease intraocular
pressure. Beta-blocker drops (timolol), carbonic anhydrase inhibitors
(acetazolamide), cholinergic stimulating drops (pilocarpine),
hyperosmotic agents (osmoglyn), and alpha-adrenergic agonists
(apraclonidine) may be employed prior to laser or surgical iridotomy (see
also Chap. 2).
Open-Angle Glaucoma
Long-term ophthalmic evaluation
and treatment with medications and laser or surgery.
Clinical Pearls
1. A high index of suspicion
must be maintained, since associated complaints such as nausea, vomiting,
and headache may obscure the diagnosis.
2. Open-angle glaucoma usually
causes no symptoms other than gradual loss of vision.
3. Congenital glaucoma is rare.
However, because of prognosis if diagnosis is delayed, consider
congenital glaucoma in infants and children with any of the following:
tearing, photophobia, enlarged eyes, cloudy corneas.
4. Asymmetric cupping, enlarged
cups, and elevated intraocular pressure are hallmarks of open-angle
glaucoma.
|
|
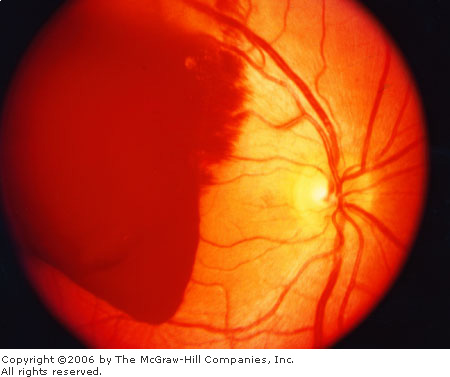
Subhyaloid Hemorrhage in Subarachnoid Hemorrhage
(SAH)
Associated Clinical Features
Subhyaloid hemorrhage appears as
extravasated blood beneath the retinal layer (Fig. 3.18). These are often
described as "boat-shaped" hemorrhages to distinguish them from
the "flame-shaped" hemorrhages on the superficial retina. They
may occur as a result of blunt trauma but are perhaps best known as a
marker for subarachnoid hemorrhage (SAH). In SAH, the hemorrhages appear
as a "puff" of blood emanating from the central disk.
|
|
|
|
|
Subhyaloid
Hemorrhage Subhyaloid
hemorrhage seen on funduscopic examination in a patient with
subarachnoid hemorrhage. (From Edlow and Caplan: Primary care:
Avoiding pitfalls in the diagnosis of subarachnoid hemorrhage. N
Engl J Med 2000; 342:29–36, with permission.)
|
|
Differential Diagnosis
SAH, shaken impact syndrome,
hypertensive retinopathy, and retinal hemorrhage should all be considered
and aggressively evaluated.
Emergency Department Treatment
and Disposition
No specific treatment is required
for subhyaloid hemorrhage. Treatment is dependent on the underlying
etiology. Appropriate specialty referral should be made in all cases.
Clinical Pearl
1. A funduscopic examination
looking for subhyaloid hemorrhage should be included in all patients with
severe headache, unresponsive pediatric patients, or those with altered
mental status.
|
|