Dinoprost Tromethamine Injection
» Dinoprost Tromethamine Injection is a sterile solution of Dinoprost Tromethamine in Water for Injection. It may contain a suitable preservative, such as benzyl alcohol. It contains not less than 90.0 percent and not more than 110.0 percent of the labeled amount of dinoprost (C20H34O5).
Caution—Extreme care should be exercised when handling dinoprost tromethamine as it is readily absorbed through the skin; accidental spillage on the skin should be washed off immediately with soap and water.
Packaging and storage—
Preserve in single-dose or in multiple-dose containers, preferably of Type I glass.
Labeling—
Label it to indicate that it is for veterinary use only.
Identification—
The retention time of the derivatized dinoprost peak in the chromatogram of the Assay preparation corresponds to that of the derivatized dinoprost peak in the Standard preparation, both relative to the internal standard, obtained as directed in the Assay.
Bacterial endotoxins  85
85 —
It contains not more than 8 USP Endotoxin Units per 1.0 mg of dinoprost.
—
It contains not more than 8 USP Endotoxin Units per 1.0 mg of dinoprost.
Sterility  71
71 —
It meets the requirements when tested as directed for Membrane Filtration under Test for Sterility of the Product to be Examined.
—
It meets the requirements when tested as directed for Membrane Filtration under Test for Sterility of the Product to be Examined.
pH  791
791 :
between 7.0 and 9.0.
:
between 7.0 and 9.0.
Other requirements—
It meets the requirements under Injections  1
1 .
.
Assay—
Mobile phase—
Prepare a solution consisting of methylene chloride, 1,3-butanediol, and water (496:3.5:0.25).
Internal standard solution—
Prepare a solution in Mobile phase containing about 0.75 ng of guaifenesin per mL.
Reagent preparations—
A—
Prepare a solution containing about 10 mg of 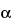 -bromo-2¢-acetonaphthone per mL of acetonitrile. Use a freshly prepared solution.
-bromo-2¢-acetonaphthone per mL of acetonitrile. Use a freshly prepared solution.
B—
Prepare a solution containing 5 µL of diisopropylethylamine per mL of acetonitrile. Use a freshly prepared solution.
C
—Prepare a citrate buffer solution by dissolving 10.5 g of citric acid monohydrate in about 75 mL of water and adding 5 N sodium hydroxide until a pH of 4.0 is obtained. Dilute with water to 100 mL, and mix.
Diluent—
Use Sterile Water for Injection containing 0.945% of benzyl alcohol.
Standard preparation—
Dissolve an accurately weighed quantity of USP Dinoprost Tromethamine RS in Diluent to obtain a solution having a known concentration of about 0.67 mg of dinoprost tromethamine per mL (Standard stock preparation). Transfer 1.0 mL of this solution to a suitable container, and add 1.0 mL of Reagent C and 20.0 mL of methylene chloride. Shake and centrifuge. Transfer 5.0 mL of the lower layer into a suitable container, and evaporate with the aid of nitrogen to dryness. Wash the inside of the container with 200 µL of Reagent A. Swirl to dissolve, add 100 µL of Reagent B, and mix. Allow the solution to stand for about 1 hour at room temperature, evaporate to dryness, add 4.0 mL of Internal standard solution, and mix to obtain a Standard preparation having a known concentration of about 0.0419 mg of USP Dinoprost Tromethamine RS per mL.
Assay preparation—
Dilute and mix an accurately measured volume of Injection with Diluent to obtain a solution having a known concentration of about 0.5 mg of dinoprost per mL (Assay stock preparation). Proceed as directed for Standard preparation, beginning with “Transfer 1.0 mL of this solution.”
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm × 30-cm column that contains packing L3. The flow rate is about 1.5 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the resolution, R, between the dinoprost tromethamine and internal standard peaks is not less than 10, and the relative standard deviation for replicate injections is not more than 2.0%.
)—
The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm × 30-cm column that contains packing L3. The flow rate is about 1.5 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the resolution, R, between the dinoprost tromethamine and internal standard peaks is not less than 10, and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Inject equal volumes (about 20 µL) of the Assay preparation and the Standard preparation into the chromatograph, record the chromatograms, and measure the peak responses at equivalent retention times. The relative retention times are about 0.4, 0.5, 1.0, and 1.2 for the internal standard, the 15-R epimer, dinoprost tromethamine, and the 5,6-trans isomer, respectively. Calculate the quantity, in mg, of C20H34O5 in each mL of the Injection taken by the formula:
(354.48/475.62)(DC)(RU / RS)
in which 354.48 and 475.62 are the molecular weights of dinoprost and dinoprost tromethamine, respectively; D is the dilution factor used in preparing the Assay stock preparation; C is the concentration, in mg per mL, of USP Dinoprost Tromethamine RS in the Standard stock preparation; and RU and RS are the ratios of the responses for the dinoprost tromethamine and internal standard peaks obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Ian DeVeau, Ph.D.
Director, Veterinary Drugs and Radiopharmaceuticals 1-301-816-8178 |
(VET05) Veterinary Drugs 05 |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
|
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance | |
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance |
USP32–NF27 Page 2171
Pharmacopeial Forum: Volume No. 33(5) Page 908
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.
