Almond Oil
Almond Oil.
Almond Oil
» Almond Oil is the refined fixed oil obtained by expression from the kernels of varieties of Prunus dulcis (Miller) D.A. Webb (formerly known as Prunus amygdalus Batsch) (Fam. Rosaceae) except for Prunus dulcis (Miller) D.A. Webb var. amara (De Candolle) Focke. It may contain suitable antioxidants.
Packaging and storage—
Preserve in tight, light-resistant, and well-filled containers. No storage requirements specified.
Labeling—
Label it to indicate the name and quantity of any added antioxidants.
Specific gravity  841
841 :
between 0.910 and 0.915.
:
between 0.910 and 0.915.
Identification—
It meets the requirements of the test for Fatty acid composition.
Acid value  401
401 :
not more than 0.5.
:
not more than 0.5.
Peroxide value  401
401 :
not more than 5.0.
:
not more than 5.0.
Unsaponifiable matter  401
401 :
not more than 0.9%.
:
not more than 0.9%.
Fatty acid composition—
Almond Oil exhibits the following composition profiles of fatty acids, as determined in the section Fatty Acid Composition under Fats and Fixed Oils  401
401 :
:
| Carbon-Chain Length | Number of Double Bonds | Percentage (%) |
| <16 | 0 | |
| 16 | 0 | 4.0–9.0 |
| 17 | 0 | |
| 18 | 0 | |
| 20 | 0 | |
| 22 | 0 | |
| 24 | 0 | |
| 16 | 1 | |
| 17 | 1 | |
| 18 | 1 | 62.0–76.0 |
| 18 | 2 | 20.0–30.0 |
| 18 | 3 | |
| 20 | 1 | |
| 22 | 1 |
Sterol composition—
separation of the sterols fraction—
Reference solution A—
Dissolve an accurately weighed quantity of cholesterol in chloroform to obtain a solution of 5% (w/v).
Developing solvent system:
a mixture of toluene and acetone (95:5) or a mixture of hexane and ether (65:35).
Test solution A—
Weigh accurately 5 g of Almond Oil into a 250-mL flask. Add 50 mL of 2 N alcoholic potassium hydroxide, and heat to gentle boiling with continuous vigorous stirring until saponification takes place (the solution becomes clear). Continue heating for a further 20 minutes, and add 50 mL of water from the top of the condenser. Cool the flask to approximately 30 . Transfer the contents of the flask to a 500-mL separating funnel with several rinses of water, amounting in all to about 50 mL. Add approximately 80 mL of ether, shake vigorously for approximately 30 seconds, and allow to settle. [note—Any emulsion can be destroyed by adding small quantities of ethyl or methyl alcohol by means of a spray.] Separate the lower aqueous phase, and collect it into a second separating funnel. Perform two further extractions on the water–alcohol phase in the same way using 60 to 70 mL of ether on each occasion. Pool the ether extracts into a single separating funnel, and wash with water, 50 mL at a time, until the wash water is no longer alkaline to phenolphthalein. Dry the ether phase with anhydrous sodium sulfate, and filter on anhydrous sodium sulfate into a previously weighed 250-mL flask, washing the funnel and filter with small quantities of ether. Distill the ether down to a few mL, and bring to dryness under a slight vacuum or in a stream of nitrogen. Complete drying at 100
. Transfer the contents of the flask to a 500-mL separating funnel with several rinses of water, amounting in all to about 50 mL. Add approximately 80 mL of ether, shake vigorously for approximately 30 seconds, and allow to settle. [note—Any emulsion can be destroyed by adding small quantities of ethyl or methyl alcohol by means of a spray.] Separate the lower aqueous phase, and collect it into a second separating funnel. Perform two further extractions on the water–alcohol phase in the same way using 60 to 70 mL of ether on each occasion. Pool the ether extracts into a single separating funnel, and wash with water, 50 mL at a time, until the wash water is no longer alkaline to phenolphthalein. Dry the ether phase with anhydrous sodium sulfate, and filter on anhydrous sodium sulfate into a previously weighed 250-mL flask, washing the funnel and filter with small quantities of ether. Distill the ether down to a few mL, and bring to dryness under a slight vacuum or in a stream of nitrogen. Complete drying at 100 for approximately 15 minutes, and then weigh after cooling in a desiccator. Dissolve the unsaponifiables so obtained in chloroform to prepare a solution having a concentration of approximately 5%.
for approximately 15 minutes, and then weigh after cooling in a desiccator. Dissolve the unsaponifiables so obtained in chloroform to prepare a solution having a concentration of approximately 5%.
Test solution B—
Treat 5 g of canola oil in the same way as prescribed for Almond Oil in Test solution A, beginning with “Add 50 mL of 2 N alcoholic potassium hydroxide”.
Test solution C—
Treat 5 g of sunflower oil in the same way as prescribed for Almond Oil in Test solution A, beginning with “Add 50 mL of 2 N alcoholic potassium hydroxide”.
Procedure—
Immerse the thin-layer chromatographic plate (see Chromatography  621
621 ), 20-cm × 20-cm silica gel on polyester with a layer thickness of 200 µm and particle size of 5–17 µm, completely in the 0.2 N alcoholic potassium hydroxide for 10 seconds, then allow to dry in a fume cupboard for 2 hours, and finally place at 100
), 20-cm × 20-cm silica gel on polyester with a layer thickness of 200 µm and particle size of 5–17 µm, completely in the 0.2 N alcoholic potassium hydroxide for 10 seconds, then allow to dry in a fume cupboard for 2 hours, and finally place at 100 for 1 hour. [note—Remove from the validated heating device, and keep the plate in a desiccator until required for use. The plates must be used within 15 days. Thin-layer chromatographic plates without requiring the preconditioning are also commercially available.] Use a separate plate for each test solution.
for 1 hour. [note—Remove from the validated heating device, and keep the plate in a desiccator until required for use. The plates must be used within 15 days. Thin-layer chromatographic plates without requiring the preconditioning are also commercially available.] Use a separate plate for each test solution.
Place a mixture of toluene and acetone (95:5) or a mixture of hexane and ether (65:35) in the chamber to a depth of approximately 1 cm. Close the chamber with the appropriate cover, and leave for at least 30 minutes. Strips of filter paper dipping into the eluent may be placed on the internal surfaces of the chamber. [note—The developing mixture should be replaced for every test to ensure reproducible elution conditions.] Apply 0.3 mL of Test solution A approximately 2 cm from the lower edge in a streak which is as thin and as uniform as possible. In line with the streak place 2 to 3 µL of Reference solution A at one end of the plate. Develop the chromatograms in an equilibrated chamber with a Developing solvent system until the solvent front reaches approximately 1 cm from the upper edge of the plate. Remove the plate from the developing chamber, and evaporate the solvent under a current of hot air [note—Avoid excessive heat.] or by leaving the plate for a short while under a hood. Spray the plate with a 0.2% alcoholic solution of 2,7-dichlorofluorescein, and examine in UV light at 254 nm. [note—The plates pretreated with UV indicator are also commercially available and used equivalently.] In each of the plates, mark the limits of the sterol band identified through being aligned with the stain obtained from Reference solution A along the edges of the fluorescence, and additionally include the area of the zones 2 to 3 mm above and below the visible zones corresponding to Reference solution A. Remove the silica gel in the marked area into a filter funnel with a G3 porous septum. Add 10 mL of hot chloroform, mix carefully with the metal spatula, filter under vacuum, and collect the filtrate in the conical flask attached to the filter funnel. Wash the residue in the funnel three times with ether, about 10 mL each time, and collect the filtrate in the same flask attached to the funnel. Evaporate the filtrate to a volume of 4 to 5 mL, transfer the residual solution to a previously weighed 10-mL test tube with a tapering bottom and a sealing stopper, and evaporate to dryness by mild heating in a gentle stream of nitrogen. Dissolve the residue in a few drops of acetone, and evaporate again to dryness. Place at 105 for approximately 10 minutes, and allow to cool in a desiccator, and weigh.
for approximately 10 minutes, and allow to cool in a desiccator, and weigh.
Treat Test solution B and Test solution C the same way as prescribed for Test solution A.
determination of the sterols—
Test solution D—
To the test tube containing the sterol fraction separated from Almond Oil by thin-layer chromatography add a freshly prepared mixture of anhydrous pyridine, hexamethyldisilazane, and chlorotrimethylsilane (9:3:1) [note—This reagent is also commercially available and used equivalently.] in the ratio of 50 µL for every mg of sterols, avoiding any uptake of moisture. Stopper the test tube, and shake carefully until the sterols are completely dissolved. Allow it to stand for at least 15 minutes at ambient temperature, and centrifuge for a few minutes if necessary. Use the supernatant. [note—The slight opalescence which may form is normal and does not cause an anomaly. However, the formation of a white floc or the appearance of a pink color is indicative of the presence of moisture or deterioration of the reagent. If these occur, the test must be repeated.]
Reference solution E—
To 9 parts of the sterols separated from canola oil by thin-layer chromatography add 1 part of cholesterol. Treat the mixture in the same way as prescribed under the Test solution D.
Reference solution F—
Treat the sterols separated from sunflower oil by thin-layer chromatography in the same way as prescribed under the Test solution D.
Chromatographic system (see Chromatography  621
621 )—
The gas chromatograph is equipped with a flame-ionization detector and a glass or fused-silica capillary column of length 20 to 30 m, internal diameter 0.25 to 0.32 mm, entirely coated with a 0.10 to 0.30-µm layer of stationary phase G27 or G36. The injection port temperature is maintained at 280
)—
The gas chromatograph is equipped with a flame-ionization detector and a glass or fused-silica capillary column of length 20 to 30 m, internal diameter 0.25 to 0.32 mm, entirely coated with a 0.10 to 0.30-µm layer of stationary phase G27 or G36. The injection port temperature is maintained at 280 , the detector temperature is maintained at 290
, the detector temperature is maintained at 290 , and the column temperature is maintained at 260 ± 5
, and the column temperature is maintained at 260 ± 5 . The carrier gas is either helium with a linear velocity of 20 to 35 cm per second or hydrogen with a linear velocity of 30 to 50 cm per second. A split ratio of 1:50 to 1:100 is used. Chromatograph Reference solution E and Reference solution F, and record the peak responses as directed for Procedure: the retention time should be 20 ± 5 minutes for
. The carrier gas is either helium with a linear velocity of 20 to 35 cm per second or hydrogen with a linear velocity of 30 to 50 cm per second. A split ratio of 1:50 to 1:100 is used. Chromatograph Reference solution E and Reference solution F, and record the peak responses as directed for Procedure: the retention time should be 20 ± 5 minutes for 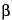 -sitosterol, and all the sterols present must be separated. [note—For peak identification purposes, the chromatogram obtained with Reference solution E shows four principal peaks corresponding to cholesterol, brassicasterol, campesterol, and
-sitosterol, and all the sterols present must be separated. [note—For peak identification purposes, the chromatogram obtained with Reference solution E shows four principal peaks corresponding to cholesterol, brassicasterol, campesterol, and 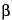 -sitosterol; and the chromatogram obtained with Reference solution F shows four principal peaks corresponding to campesterol, stigmasterol,
-sitosterol; and the chromatogram obtained with Reference solution F shows four principal peaks corresponding to campesterol, stigmasterol, 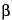 -sitosterol, and D7-stigmastenol. The retention times of the sterols with reference to
-sitosterol, and D7-stigmastenol. The retention times of the sterols with reference to 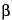 -sitosterol are given in Table 1.]
-sitosterol are given in Table 1.]
Table 1. Relative Retention Times of Sterols for Two Different Columns
| Identification | G36 column | G27 column |
| Cholesterol | 0.67 | 0.63 |
| Brassicasterol | 0.73 | 0.71 |
| 24-Methylene-cholesterol | 0.82 | 0.80 |
| Campesterol | 0.83 | 0.81 |
| Campestanol | 0.85 | 0.82 |
| Stigmasterol | 0.88 | 0.87 |
| D7-Campesterol | 0.93 | 0.92 |
| D5,23-Stigmastadienol | 0.95 | 0.95 |
| Clerosterol | 0.96 | 0.96 |
| 1.00 | 1.00 | |
| Sitostanol | 1.02 | 1.02 |
| D5-Avenasterol | 1.03 | 1.03 |
| D5,24-Stigmastadienol | 1.08 | 1.08 |
| D7-Stigmastenol | 1.12 | 1.12 |
| D7-Avenasterol | 1.16 | 1.16 |
Procedure—
Separately inject equal volumes (about 1 µL) of Test solution D, Reference solution E, and Reference solution F into the chromatograph, record the chromatograms, and measure the peak areas for the sterols. Calculate the percentage of each individual sterol in the sterol fraction of Almond Oil taken by the formula:
100(A / S)
in which A is the area of the peak due to the sterol component to be determined, and S is the sum of the areas of the peaks due to the components indicated in Table 1. Almond Oil exhibits the following composition profiles of sterols.
| Component | Percentage (%) |
| Cholesterol | |
| Brassicasterol | |
| Campesterol | |
| Stigmasterol | |
| 73.0–87.0 | |
| D5-Avenasterol | |
| D7-Stigmastenol | |
| D7-Avenasterol |
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Hong Wang, Ph.D.
Scientist 1-301-816-8351 |
(EM205) Excipient Monographs 2 |
USP32–NF27 Page 1160
Pharmacopeial Forum: Volume No. 32(4) Page 1147
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.