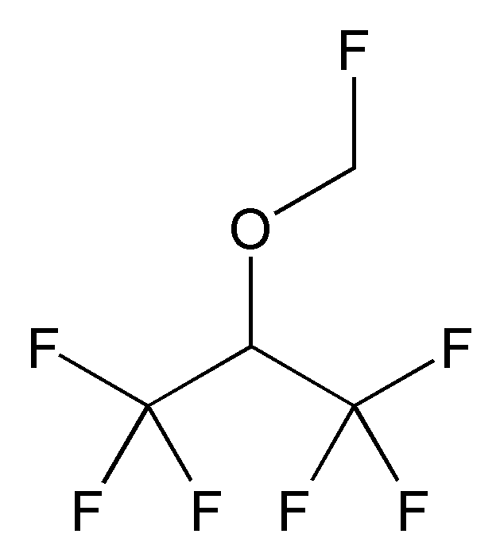
Sevoflurane
» Sevoflurane contains not less than 99.97 percent and not more than 100.00 percent of C4H3F7O.
Packaging and storage—
Preserve in tight, light-resistant containers. Store at controlled room temperature. Replace the cap securely after each use.
USP Reference standards  11
11 —
—
USP Sevoflurane RS .
.
USP Sevoflurane Related Compound A RS .
.
USP Sevoflurane Related Compound B RS .
.
USP Sevoflurane Related Compound C RS .
.
USP Sevoflurane RS
 .
.
USP Sevoflurane Related Compound A RS
 .
.
USP Sevoflurane Related Compound B RS
 .
. USP Sevoflurane Related Compound C RS
 .
.
Identification, Infrared Absorption—
The IR absorption spectrum of Sevoflurane, obtained using a gas cell, exhibits maxima only at the same wavelengths as that of a similar preparation of USP Sevoflurane RS.
Refractive index  831
831 :
between 1.2745 and 1.2760, at 20
:
between 1.2745 and 1.2760, at 20 .
.
Acidity or alkalinity—
Transfer 20.0 mL of Sevoflurane and 20.0 mL of carbon dioxide-free water to a separatory funnel, shake for 3 minutes, and allow the layers to separate: the aqueous layer requires not more than 0.10 mL of 0.010 N sodium hydroxide or not more than 0.60 mL of 0.010 N hydrochloric acid for neutralization, bromocresol purple TS being used as the indicator.
Water, Method I  921
921 :
not more than 0.1%.
:
not more than 0.1%.
Limit of fluoride—
[note—Use plastic utensils throughout this test.]
Buffer solution—
Transfer 110 g of sodium chloride and 1 g of sodium citrate to a 2000-mL volumetric flask, and dissolve in 700 mL of water. Carefully add 150 g of sodium hydroxide, and shake to dissolve. Cool to room temperature, and carefully add 450 mL of glacial acetic acid while stirring. Cool, add 600 mL of isopropyl alcohol, dilute with water to volume, and mix. [note—The pH of this solution is between 5.0 and 5.5. This solution may be used for 6 weeks when stored at room temperature.]
Solution A—
Transfer about 221 mg of sodium fluoride, previously dried at 150 for 4 hours and accurately weighed, to a 100-mL volumetric flask. Add about 20 mL of water, and mix to dissolve. Add 1.0 mL of 0.01 N sodium hydroxide, and dilute with water to volume. Each mL of this solution contains 1 mg of fluoride. Store in a tightly closed plastic container. [note—This solution may be used for 2 weeks when stored in a refrigerator.]
for 4 hours and accurately weighed, to a 100-mL volumetric flask. Add about 20 mL of water, and mix to dissolve. Add 1.0 mL of 0.01 N sodium hydroxide, and dilute with water to volume. Each mL of this solution contains 1 mg of fluoride. Store in a tightly closed plastic container. [note—This solution may be used for 2 weeks when stored in a refrigerator.]
Standard stock solutions—
Quantitatively transfer accurately measured volumes of Solution A to separate 100-mL volumetric flasks, and dilute with water to obtain solutions having known concentrations of about 5, 2, 0.5, and 0.2 µg of fluoride per mL.
Standard solutions—
Transfer 25.0 mL of each of the Standard stock solutions to separate 50-mL volumetric flasks, dilute with Buffer solution to volume, and mix.
Test solution—
Pipet 50.0 mL of Sevoflurane and 50.0 mL of water into a separatory funnel, shake vigorously for 3 minutes, and allow the liquids to separate completely. Transfer 25.0 mL of the aqueous top layer to a 50-mL volumetric flask, dilute with Buffer solution to volume, and mix.
Procedure—
Concomitantly measure the potentials, in mV, of the Standard stock solutions, Standard solutions, and Test solution with a pH meter (see pH  791
791 ) capable of a minimum reproducibility of ±0.2 mV and equipped with a fluoride-specific ion-indicating electrode and a glass-sleeved calomel reference electrode. [note—When taking measurements, transfer the solution under test to a 100-mL beaker containing a polytef-coated stirring bar, and immerse the electrodes. Allow to stir on a magnetic stirrer having an insulated top until equilibrium is attained in about 2 to 3 minutes, and record the potential. Rinse the electrodes with the Buffer solution, and dry, taking care to avoid damaging the crystal of the specific-ion electrode. A satisfactory response is achieved if the difference between the potentials obtained with the Standard stock solutions having fluoride concentrations of 5 and 0.5 µg per mL is in the range between 50 and 60 mV.] Plot the logarithms of the fluoride concentrations, in µg per mL, of the Standard solutions versus potentials, in mV. From the graph so obtained and the measured potential of the Test solution, determine the concentration, in µg per mL, of fluoride in the Test solution: not more than 2 µg per mL is found.
) capable of a minimum reproducibility of ±0.2 mV and equipped with a fluoride-specific ion-indicating electrode and a glass-sleeved calomel reference electrode. [note—When taking measurements, transfer the solution under test to a 100-mL beaker containing a polytef-coated stirring bar, and immerse the electrodes. Allow to stir on a magnetic stirrer having an insulated top until equilibrium is attained in about 2 to 3 minutes, and record the potential. Rinse the electrodes with the Buffer solution, and dry, taking care to avoid damaging the crystal of the specific-ion electrode. A satisfactory response is achieved if the difference between the potentials obtained with the Standard stock solutions having fluoride concentrations of 5 and 0.5 µg per mL is in the range between 50 and 60 mV.] Plot the logarithms of the fluoride concentrations, in µg per mL, of the Standard solutions versus potentials, in mV. From the graph so obtained and the measured potential of the Test solution, determine the concentration, in µg per mL, of fluoride in the Test solution: not more than 2 µg per mL is found.
Limit of nonvolatile residue—
Transfer 10.0 mL of Sevoflurane to an accurately weighed evaporating dish, evaporate to dryness on a steam bath, and dry the residue at 105 for 2 hours: the weight of the residue does not exceed 1.0 mg.
for 2 hours: the weight of the residue does not exceed 1.0 mg.
Limit of peroxide—
Titanium tetrachloride solution—
Separately cool 1.0 mL of 6 N hydrochloric acid and 1.0 mL of titanium tetrachloride in small beakers surrounded by crushed ice. Add titanium tetrachloride dropwise to the chilled acid, and allow to dissolve. After complete dissolution, dilute with 6 N hydrochloric acid to 100 mL, and mix.
Standard stock solution—
Dilute 1 mL of 30 percent hydrogen peroxide with water to 400 mL.
Standard solution—
Transfer 15.0 mL of the Standard stock solution to a 1000-mL volumetric flask, dilute with water to volume, and mix. Transfer 1.0 mL of the solution so obtained and 5.0 mL of Titanium tetrachloride solution to a 10-mL volumetric flask, dilute with water to volume, and mix.
Test solution—
Transfer 50.0 mL of Sevoflurane and 5.0 mL of Titanium tetrachloride solution to a separatory funnel, shake vigorously, allow the layers to separate, drain, and discard the lower layer. Carefully collect the top layer in a 10-mL volumetric flask, dilute with water to volume, and mix.
Procedure—
Concomitantly determine the absorbances of the Standard solution and the Test solution at a wavelength of about 410 nm, with a suitable spectrophotometer, using a 1-cm cell and a mixture of Titanium tetrachloride solution and water (1:1) as the blank. Calculate the peroxide concentration, in µg per mL, in the portion of Sevoflurane taken by the formula:
0.22(AU / AS)
in which AU and AS are the absorbances obtained from the Test solution and the Standard solution, respectively: not more than 0.22 µg per mL is found.
Related compounds—
Internal standard solution—
Use dimethoxymethane.
Ethylene dichloride identification solution—
Transfer 2.0 mL of Sevoflurane to a vial, and seal with a cap and septum. Using a microsyringe, add 20 µL of ethylene dichloride through the septum of the vial, and mix thoroughly.
Sevoflurane related compounds stock solution—
Transfer 20 mL of Sevoflurane to a 40-mL vial with a septum lid. Add 20 µL each of USP Sevoflurane Related Compound A RS, USP Sevoflurane Related Compound B RS, and USP Sevoflurane Related Compound C RS to the vial, and mix thoroughly.
Related compounds identification solution—
Transfer 1.0 mL of Ethylene dichloride identification solution to a 10-mL volumetric flask, and dilute with Sevoflurane to volume. Transfer 2 mL of this solution and 5 mL of the Sevoflurane related compounds stock solution to a 50-mL volumetric flask, dilute with Sevoflurane to volume, and mix thoroughly.
Standard solutions—
Prepare in duplicate, proceeding for each as follows. Transfer 2.0 mL of ethylene dichloride to a screw-capped vial, immediately seal with a cap and septum, and place on a balance. Using a microsyringe, transfer about 20 µL of USP Sevoflurane RS, accurately measured, to the vial by inserting the syringe needle through the septum. Record the quantity, in mg, of USP Sevoflurane RS added. Using the same method, transfer about 20 µL of Internal standard solution to the vial, and record the quantity, in mg, of the solution added.
Control standard solution—
Place a 40-mL vial with a septum lid on an analytical balance, and tare out the weight. Add 30 mL of ethylene dichloride to the vial, and seal tightly. Record the weight of the ethylene dichloride, and tare. Using a microsyringe, add 20 µL of the USP Sevoflurane RS through the septum of the vial, record the weight, and mix thoroughly. Transfer 1.0 mL of this solution to a 100-mL volumetric flask, and dilute with ethylene dichloride to volume.
Test solution—
Transfer 20.0 mL of Sevoflurane to a vial, and insert the stopper. Using a microsyringe, add 5 µL of Internal standard solution, accurately measured, to the vial.
Chromatographic system (see Chromatography  621
621 )—
The gas chromatograph is equipped with a flame-ionization detector and a 0.32-mm × 30-m fused-silica capillary column coated with a 3.0-µm film of liquid phase G43. Prior to use, condition the column overnight at a temperature of 250
)—
The gas chromatograph is equipped with a flame-ionization detector and a 0.32-mm × 30-m fused-silica capillary column coated with a 3.0-µm film of liquid phase G43. Prior to use, condition the column overnight at a temperature of 250 . The chromatograph is programmed as follows. The column temperature is initially maintained at 40
. The chromatograph is programmed as follows. The column temperature is initially maintained at 40 for 10 minutes, then increased at a rate of 10
for 10 minutes, then increased at a rate of 10 per minute to 200
per minute to 200 , and maintained at 200
, and maintained at 200 for at least 14 minutes. The injection port temperature is maintained at 200
for at least 14 minutes. The injection port temperature is maintained at 200 . The detector temperature is maintained at 225
. The detector temperature is maintained at 225 . The split ratio is 1:20. Helium is used as the carrier gas, flowing at a rate of about 1.0 mL per minute. The make-up gas flow rate is about 20 mL per minute. Chromatograph one of the Standard solutions, and record the chromatograms as directed for Procedure: the column efficiency is not less than 6000 theoretical plates; and the relative standard deviation for replicate injections, determined from the peak area ratio of sevoflurane to the internal standard, is not more than 3.0%. Chromatograph the Related compounds identification solution, record the chromatograms as directed for Procedure, and identify the peaks using the relative retention times given in Table 1: the resolution, R, between sevoflurane related compound C and ethylene dichloride is not less than 2.0.
. The split ratio is 1:20. Helium is used as the carrier gas, flowing at a rate of about 1.0 mL per minute. The make-up gas flow rate is about 20 mL per minute. Chromatograph one of the Standard solutions, and record the chromatograms as directed for Procedure: the column efficiency is not less than 6000 theoretical plates; and the relative standard deviation for replicate injections, determined from the peak area ratio of sevoflurane to the internal standard, is not more than 3.0%. Chromatograph the Related compounds identification solution, record the chromatograms as directed for Procedure, and identify the peaks using the relative retention times given in Table 1: the resolution, R, between sevoflurane related compound C and ethylene dichloride is not less than 2.0.
Procedure—
Separately inject equal volumes (about 2 µL) of the Standard solutions, the Test solution, the Control standard solution, and the Ethylene dichloride identification solution into the chromatograph, record the chromatograms, and measure the areas of the major peaks. Calculate the response factor for each of the Standard solutions by the formula:
(WI / WS)RS
in which WI is the weight, in mg, of the internal standard in the Standard solution; WS is the weight, in mg, of USP Sevoflurane RS in the Standard solution; and RS is the response ratio of the sevoflurane peak to that of the internal standard obtained from the Standard solutions: the response factors for the duplicate Standard solutions do not differ by more than 3.0% from their average. Calculate the quantity, in µg per g, of each impurity in the portion of Sevoflurane taken by the formula:
250(0.859/1.525)(Ri / FR)(1/F)
in which 0.859 and 1.525 are the specific gravities of the internal standard and sevoflurane, respectively; Ri is the response ratio of the impurity peak to that of the internal standard obtained from the Test solution; FR is the average response factor obtained as directed above; and F is the respective relative response factor for the impurities (see Table 1 for values): not more than 25 µg per g of sevoflurane related compound A, not more than 100 µg per g of any other single impurity, and not more than 300 µg per g of total impurities is found. [note—Do not include sevoflurane, the internal standard, or any peak identified as solvent carryover (ethylene dichloride). Also, disregard any peak with an area less than 30% of the average area of the principal peak in the chromatogram obtained with the Control standard solution.]
Table 1
| Compound | Relative Retention Time |
Relative Response Factor (F) |
| Sevoflurane related compound A | 0.57 | 1.0 |
| Sevoflurane related compound B | 0.62 | 1.0 |
| Sevoflurane | 0.74 | — |
| Internal standard (dimethoxymethane) | 1.0 | — |
| Ethylene dichloride | 1.69 | — |
| Sevoflurane related compound C | 1.71 | 0.46 |
| Unknown impurities | — | 1 |
Assay—
Using the results from the test for Related compounds, calculate the percentage of C4H3F7O in the volume of Sevoflurane taken by subtracting the sum of percentages for all impurities found from 100.00%.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Daniel K. Bempong, Ph.D.
Senior Scientist 1-301-816-8143 |
(MDPS05) Monograph Development-Pulmonary and Steroids |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 3552
Pharmacopeial Forum: Volume No. 33(5) Page 940
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.